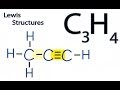
Lewis Structures and Valence Electrons
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Aiden Montgomery
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are available for the C3H4 molecule?
18
12
16
14
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the first Lewis structure, how many double bonds are present?
Two
Three
None
One
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bonding arrangement in the second Lewis structure?
Two double bonds
One double bond and two single bonds
One triple bond and one single bond
Three single bonds
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the second Lewis structure, how many bonds does each carbon atom have?
Four
Three
Two
Five
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the shape of the third Lewis structure?
Tetrahedral
Ring
Bent
Linear
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many single bonds are present in the third Lewis structure?
Two
Three
One
Four
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the third Lewis structure, how many valence electrons does each hydrogen atom have?
Four
Three
Two
One
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Gametogenesis
Interactive video
•
9th - 10th Grade

11 questions
Fundamentals of Chemistry Concepts
Interactive video
•
9th - 12th Grade

6 questions
CLEAN : Rail, bus workers strike in Paris over conditions, tenders to competition
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Poland: Andrzej Duda unseats Komorowski
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Bill Gates predicts end for polio
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Palestinian official gives update on coronavirus
Interactive video
•
9th - 10th Grade

6 questions
Human Body /Human Body Systems/Human Anatomy
Interactive video
•
KG - 9th Grade

11 questions
Claims and Evidence in Arguments
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade