
Lewis Structures and Valence Electrons
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Aiden Montgomery
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
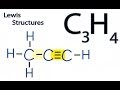
How many valence electrons are available for the C3H4 molecule?
18
12
16
14
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the first Lewis structure, how many double bonds are present?
Two
Three
None
One
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bonding arrangement in the second Lewis structure?
Two double bonds
One double bond and two single bonds
One triple bond and one single bond
Three single bonds
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the second Lewis structure, how many bonds does each carbon atom have?
Four
Three
Two
Five
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the shape of the third Lewis structure?
Tetrahedral
Ring
Bent
Linear
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many single bonds are present in the third Lewis structure?
Two
Three
One
Four
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the third Lewis structure, how many valence electrons does each hydrogen atom have?
Four
Three
Two
One
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Isotopes and Ions Concepts
Interactive video
•
9th - 10th Grade

11 questions
Methyl Groups in Organic Chemistry
Interactive video
•
9th - 10th Grade

7 questions
Lewis Structures and Electron Pairs
Interactive video
•
9th - 10th Grade

9 questions
Hydrogen Cyanide Bonding and Properties
Interactive video
•
9th - 10th Grade

6 questions
GCSE Secondary English Age 13-17 - Writing: Forms of Writing: Fiction Part 2 - Explained
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Demo in Hong Kong as China favourite Lam wins leadership
Interactive video
•
9th - 10th Grade

11 questions
Ionic Bonds and Electron Transfer
Interactive video
•
9th - 10th Grade

11 questions
CHO+ Molecular Geometry and Bonding
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

8 questions
Spartan Way - Classroom Responsible
Quiz
•
9th - 12th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

14 questions
Boundaries & Healthy Relationships
Lesson
•
6th - 8th Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

3 questions
Integrity and Your Health
Lesson
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

9 questions
FOREST Perception
Lesson
•
KG

20 questions
Main Idea and Details
Quiz
•
5th Grade
Discover more resources for Chemistry

22 questions
Unit 9 Gas Law Quiz
Quiz
•
10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

13 questions
Solubility Curves
Quiz
•
10th Grade

20 questions
momentum and impulse
Quiz
•
9th - 12th Grade

22 questions
Solubility Curve Practice
Quiz
•
10th Grade

40 questions
Unit 3 (Part 1) Chemical Equations & Reactions Review Game
Quiz
•
8th - 12th Grade

35 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

15 questions
Ionic Bonding
Quiz
•
10th - 11th Grade