
PCl6- Ion Structure and Properties
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Sophia Harris
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
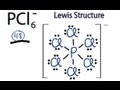
How many total valence electrons are present in the PCL6- ion?
42
48
36
54
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center of the PCL6- Lewis structure?
Nitrogen
Oxygen
Chlorine
Phosphorus
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form chemical bonds in the PCL6- structure?
12
10
16
14
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of completing octets for chlorine atoms in the PCL6- structure?
It stabilizes the molecule.
It increases the molecule's reactivity.
It changes the molecule's charge.
It decreases the molecule's size.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why can phosphorus have more than eight valence electrons in the PCL6- structure?
It is in Period 4 of the periodic table.
It is in Period 3 of the periodic table.
It is in Period 2 of the periodic table.
It is in Period 1 of the periodic table.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What notation is used to indicate that PCL6- is an ion?
Brackets with a negative sign outside
A positive sign outside the brackets
No special notation is needed
A negative sign inside the brackets
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

29 questions
Alg. 1 Section 5.1 Coordinate Plane
Quiz
•
9th Grade

22 questions
fractions
Quiz
•
3rd Grade

11 questions
FOREST Effective communication
Lesson
•
KG

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Chemistry

22 questions
Unit 9 Gas Law Quiz
Quiz
•
10th Grade

10 questions
Exploring Types of Chemical Reactions
Interactive video
•
6th - 10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Acids and Bases
Quiz
•
10th Grade

30 questions
Energy Review
Quiz
•
9th Grade

7 questions
GCSE Chemistry - Balancing Chemical Equations #4
Interactive video
•
9th - 10th Grade

20 questions
Chemistry: Classification of Matter
Quiz
•
10th Grade

40 questions
Unit 3 (Part 1) Chemical Equations & Reactions Review Game
Quiz
•
8th - 12th Grade