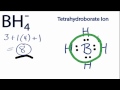
Boron and BH4- Lewis Structure
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are there in total for the BH4- ion?
9
8
7
6
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where is Boron placed in the BH4- Lewis structure?
At the top
On the side
At the center
At the bottom
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does each Hydrogen need to have a full outer shell?
2
1
3
4
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons used in the BH4- Lewis structure?
9
6
7
8
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of adding brackets around the BH4- ion in its Lewis structure?
To highlight the central atom
To indicate a positive charge
To show the ion is neutral
To denote a negative charge
Similar Resources on Wayground

7 questions
Lewis Structures and Valence Electrons
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons in N2O4 Structure
Interactive video
•
9th - 10th Grade

6 questions
Polarity of O2 Molecule Concepts
Interactive video
•
9th - 10th Grade

8 questions
Valence Electrons in C2Cl6
Interactive video
•
9th - 10th Grade

8 questions
Valence Electrons in CHF3
Interactive video
•
9th - 10th Grade

8 questions
CH2Br2 Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
NO2Cl Structure and Bonding Concepts
Interactive video
•
9th - 10th Grade

7 questions
Bromine Lewis Structure Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade