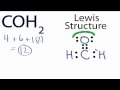
Valence Electrons in COH2 Structure
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Liam Anderson
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does Carbon have in the COH2 molecule?
8
4
2
6
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in COH2?
14
12
10
16
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where is Carbon placed in the COH2 Lewis structure?
Next to Oxygen
On the outside
Next to Hydrogen
In the middle
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do Hydrogens go on the outside in the COH2 structure?
They have the most valence electrons
They are the least electronegative
They only need two electrons to fill their shell
They are heavier than Carbon and Oxygen
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What adjustment is made to ensure Carbon has a full outer shell?
Add more valence electrons
Create a double bond with Oxygen
Remove an electron from Oxygen
Add more Hydrogen atoms
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
After forming a double bond, how many valence electrons does Oxygen have?
6
8
10
12
Similar Resources on Wayground

6 questions
Valence Electrons in PH3 Molecule
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons and Bonding in Fluorine
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons in HOH Structure
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons in IBr2- Structure
Interactive video
•
9th - 10th Grade

8 questions
Valence Electrons and Periodic Table
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in SiCl4
Interactive video
•
9th - 10th Grade

6 questions
SF2 Lewis Structure and Valence Electrons
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade