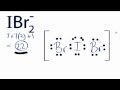
Valence Electrons in IBr2- Structure
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Liam Anderson
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many total valence electrons are present in the IBr2- ion?
20
21
22
23
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which element is placed at the center of the IBr2- Lewis structure?
Fluorine
Chlorine
Iodine
Bromine
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form chemical bonds between the atoms in IBr2-?
4
6
2
8
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons around each bromine atom in the IBr2- structure?
9
8
7
6
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why can iodine hold more than eight valence electrons in the IBr2- structure?
It is in period 6
It is in period 3
It is in period 4
It is in period 5
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What notation is used to indicate that IBr2- is an ion?
Parentheses
Brackets
Curly braces
Angle brackets
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade