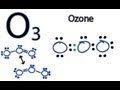
Ozone Lewis Structures and Resonance
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Lucas Foster
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are there in total for ozone (O3)?
6
12
18
24
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in forming the Lewis structure for ozone?
Placing electrons around each oxygen atom
Forming a double bond immediately
Calculating the total number of valence electrons
Drawing a single bond between all oxygen atoms
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
After placing electrons between the oxygen atoms, what is checked next?
The fulfillment of the octet rule
The molecular shape
The presence of double bonds
The total number of electrons used
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What adjustment is made to fulfill the octet rule for the central oxygen atom?
Forming a double bond by sharing electrons
Removing electrons from the outer atoms
Changing the molecular shape
Adding more electrons to the central atom
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a resonance structure?
A structure with only single bonds
A structure with more than one correct Lewis structure
A structure with an incorrect number of electrons
A structure that does not follow the octet rule
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is resonance represented in the Lewis structure of ozone?
By using different colors for bonds
By drawing both structures with an arrow between them
By using dashed lines for bonds
By changing the shape of the molecule
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is true about the bonds in ozone's resonance structures?
They are always double bonds
They are always single bonds
They can be either single or double bonds
They do not exist
Similar Resources on Wayground
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade