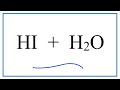
Hydroiodic Acid and Strong Acids
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Ethan Morris
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'H' in front of HI indicate about the compound?
It is a base.
It is a salt.
It is an acid.
It is a neutral compound.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for hydroiodic acid?
HCl
HI
HBr
HF
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is true about strong acids like HI?
They partially dissociate in water.
They form a precipitate in water.
They do not dissociate in water.
They completely dissociate in water.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is NOT a characteristic of strong acids?
They completely dissociate in water.
They are good conductors of electricity.
They are weak electrolytes.
They have a high concentration of H+ ions.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What ions are formed when HI dissociates in water?
H+ and OH-
H2 and I2
H+ and I-
H2O and I2
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge on the iodide ion formed from HI?
2+
1+
1-
2-
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the term 'aqueous' signify in the context of dissociated ions?
The ions are in a liquid state.
The ions are in a solid state.
The ions are dissolved in water.
The ions are in a gaseous state.
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is formed when H+ ions interact with water molecules?
Hydronium ion (H3O+)
Water molecule (H2O)
Hydroxide ion (OH-)
Iodide ion (I-)
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the hydronium ion represented in chemical notation?
H3O+
H+
H2O
OH-
Similar Resources on Wayground

11 questions
Moles and Molecular Formulas
Interactive video
•
9th - 10th Grade

6 questions
Top 5 Clips of August 31st
Interactive video
•
9th - 10th Grade

6 questions
VOICED : Environmental groups warn over toxic mud in Hungary
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : A Rio favela has to avoid alligators to collect drinking water
Interactive video
•
9th - 10th Grade

6 questions
CLEAN: Biochar: British tests refine carbon-capture solution
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Greece celebrates Independence Day
Interactive video
•
9th - 10th Grade

8 questions
TED: Can we call it a "world map" if it's missing a billion people? | Rebecca Firth
Interactive video
•
9th - 10th Grade

11 questions
IELTS Writing: Letter Writing
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade