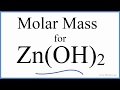
Molar Mass Calculations and Concepts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Liam Anderson
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of zinc as found on the periodic table?
99.4 grams per mole
1.01 grams per mole
16.00 grams per mole
65.38 grams per mole
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the subscript '2' in the hydroxide group affect the calculation?
It doubles the molar mass of zinc.
It applies to both oxygen and hydrogen in the group.
It only affects the oxygen atom.
It has no effect on the calculation.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of oxygen used in the calculation?
99.4 grams per mole
65.38 grams per mole
16.00 grams per mole
1.01 grams per mole
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of hydrogen used in the calculation?
99.4 grams per mole
1.01 grams per mole
16.00 grams per mole
65.38 grams per mole
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final calculated molar mass of zinc hydroxide?
1.01 grams per mole
16.00 grams per mole
65.38 grams per mole
99.4 grams per mole
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why might there be a slight difference in the final molar mass calculation?
Due to different periodic tables used.
Mistake in multiplying the hydroxide group.
Incorrect calculation of zinc's molar mass.
Because of rounding differences.
Similar Resources on Wayground

6 questions
Blizzards: The Chilling Power of Winter Storms
Interactive video
•
KG - University

11 questions
Perpetual Motion Machines and Thermodynamics
Interactive video
•
9th - 12th Grade

11 questions
Projectile Motion and Height Analysis
Interactive video
•
8th - 10th Grade

11 questions
Flint Water Crisis: Understanding the Science and Consequences
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade