Electronegativity and Molecular Polarity
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
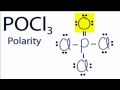
What is the primary reason the P3 molecule is not symmetrical?
It has an equal number of atoms on all sides.
It contains three chlorine atoms and one oxygen atom.
It is a linear molecule.
It has a central nitrogen atom.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does the oxygen atom in the P3 molecule have a slight negative charge?
Because it has a lower atomic number than chlorine.
Because it shares electrons equally with chlorine.
Because it is less electronegative than chlorine.
Because it is more electronegative than chlorine.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of having different electronegativities in the P3 molecule?
The molecule becomes symmetrical.
The molecule has distinct positive and negative poles.
The molecule forms a linear shape.
The molecule becomes non-polar.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following best describes the P3 molecule?
Polar due to equal distribution of electrons.
Non-polar due to equal electronegativity of atoms.
Polar due to asymmetrical structure and charge distribution.
Non-polar due to symmetrical structure.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of having poles in a molecule?
It indicates the molecule is non-polar.
It suggests the molecule is symmetrical.
It means the molecule has no charge distribution.
It confirms the molecule is polar.
Similar Resources on Wayground

8 questions
Polarity and Electronegativity of SeF4
Interactive video
•
9th - 10th Grade

2 questions
EASILY know if a Molecule is POLAR or NONPOLAR in Chemistry
Interactive video
•
10th Grade - University

3 questions
IIT/JEE Chemistry Practice #18: Intermolecular Forces
Interactive video
•
11th Grade - University

6 questions
Polarity and Structure of CS2
Interactive video
•
9th - 10th Grade

2 questions
Molecular Matters Polar and Non-Polar Solids
Interactive video
•
10th Grade - University

2 questions
Midnight Sun: Why Sun Never Sets in Alaska for Months?
Interactive video
•
KG - University

6 questions
Polar And Nonpolar Covalent Bonds: Easy Explanation With Examples - Chemistry Basics
Interactive video
•
10th Grade - University

6 questions
Understanding Chemical Bonds and Intermolecular Forces
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade