Understanding Molecular Polarity
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard

Nancy Jackson
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What determines if a molecule is polar?
The number of protons in the molecule
The size of the molecule
The speed of electron movement
The distribution of electrons within the molecule
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
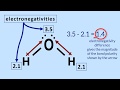
Why is water considered a polar molecule?
Because it has an equal distribution of electrons
Because oxygen pulls electrons away from hydrogen
Because oxygen and hydrogen have the same electronegativity
Because it has a symmetrical shape
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the shape of a carbon dioxide molecule?
Bent
Linear triatomic
Pyramidal
Tetrahedral
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does symmetry affect the polarity of a molecule?
Symmetry always makes a molecule polar
Symmetry increases the electronegativity difference
Symmetry has no effect on polarity
Symmetry causes bond polarities to cancel out
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the polarity of BF3 when one fluorine is replaced with chlorine?
The molecule becomes non-polar
The molecule remains the same
The molecule becomes symmetrical
The molecule becomes polar
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in determining molecular polarity using the flowchart?
Check the size of the molecule
Check if any bonds are polar
Check if the molecule is symmetrical
Check the speed of electron movement
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the polarity of HCl?
Neutral
Symmetrical
Polar
Non-polar
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade