
Rates of Reaction
Quiz
•
Chemistry
•
11th Grade
•
Medium
Georgina Casey
Used 6+ times
FREE Resource
23 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does a catalyst work in speeding up a reaction?
By lowering the activation energy
by giving them more energy
by making them more available
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which graph shows the effect of increasing temperature on the rate of reaction of calcium carbonate with dilute hydrochloric acid?
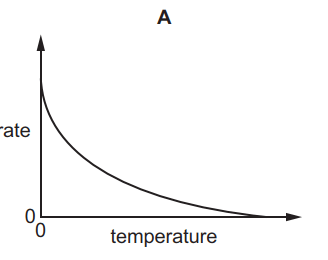
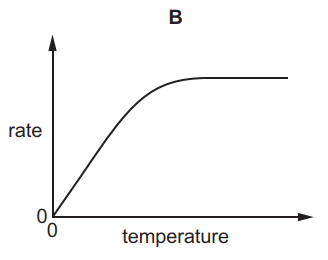
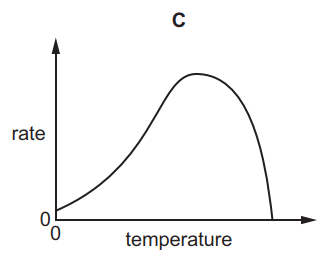
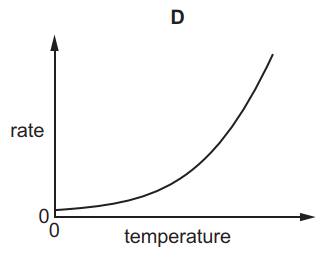
5.
MULTIPLE CHOICE QUESTION
15 mins • 1 pt

6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Create a free account and access millions of resources
Similar Resources on Quizizz

19 questions
Regents Kinetics
Quiz
•
9th - 12th Grade

25 questions
Collision Theory and Reaction Rates
Quiz
•
9th - 11th Grade

18 questions
AP Chemistry - Unit 5 - Thou Shall Not Forget
Quiz
•
9th - 12th Grade

20 questions
Rates of reaction
Quiz
•
9th - 11th Grade

20 questions
KS4 Chemistry - Rates of Reaction
Quiz
•
7th - 11th Grade

19 questions
AQA Chemistry Paper 1- Energy Changes
Quiz
•
9th - 11th Grade

20 questions
Rates of reaction and reversible reactions
Quiz
•
9th - 11th Grade

20 questions
Le Chatelier's Principle
Quiz
•
9th - 12th Grade
Popular Resources on Quizizz

15 questions
Multiplication Facts
Quiz
•
4th Grade

20 questions
Math Review - Grade 6
Quiz
•
6th Grade

20 questions
math review
Quiz
•
4th Grade

5 questions
capitalization in sentences
Quiz
•
5th - 8th Grade

10 questions
Juneteenth History and Significance
Interactive video
•
5th - 8th Grade

15 questions
Adding and Subtracting Fractions
Quiz
•
5th Grade

10 questions
R2H Day One Internship Expectation Review Guidelines
Quiz
•
Professional Development

12 questions
Dividing Fractions
Quiz
•
6th Grade



