
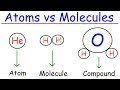
Exploring Elements, Atoms, and Compounds in Chemistry
Interactive Video
•
Science
•
6th - 10th Grade
•
Practice Problem
•
Medium
Standards-aligned
Jackson Turner
Used 35+ times
FREE Resource
Standards-aligned
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following substances is composed of atoms?
Carbon dioxide
Hydrogen gas
Water
Helium
Tags
NGSS.MS-PS1-1
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What defines a molecule?
A particle with one atom
A particle with multiple atoms
A particle with only one type of atom
A particle with different types of atoms
Tags
NGSS.MS-PS1-1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is a pure element?
Water
Sodium chloride
Carbon dioxide
Oxygen gas
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a compound?
A substance with only one type of atom
A substance with only one molecule
A substance with multiple types of atoms
A substance with no atoms
Tags
NGSS.MS-PS1-1
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do atoms and ions differ?
Atoms have more protons than electrons
Ions have equal numbers of protons and electrons
Atoms are electrically neutral
Ions are always negatively charged
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of an aluminum ion with 13 protons and 10 electrons?
-1
-3
+3
+1
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is an ionic compound?
Water
Carbon monoxide
Magnesium chloride
Sulfur hexafluoride
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

29 questions
Alg. 1 Section 5.1 Coordinate Plane
Quiz
•
9th Grade

22 questions
fractions
Quiz
•
3rd Grade

11 questions
FOREST Effective communication
Lesson
•
KG

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Science

22 questions
Phases of the moon
Quiz
•
8th Grade

20 questions
Rocks and The Rock Cycle
Quiz
•
6th Grade

15 questions
Rock Cycle
Quiz
•
6th Grade

10 questions
Exploring the Rock Cycle
Interactive video
•
6th - 8th Grade

20 questions
Flow of Energy
Quiz
•
7th Grade

12 questions
Ecological Succession
Quiz
•
7th Grade

20 questions
Waves and Wave Properties
Quiz
•
6th - 8th Grade

8 questions
Amoeba Sister Asexual vs Sexual Reproduction
Interactive video
•
8th Grade