Exploring the Conservation of Mass Principles
Interactive Video
•
Amelia Wright
•
Science
•
6th - 10th Grade
•
Hard
Standards-aligned
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the law of conservation of mass state?
Mass can be created and destroyed.
Mass can only be destroyed.
Mass can only be created.
Mass can neither be created nor destroyed.
Tags
NGSS.MS-PS1-5
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the first example, what are the reactants?
Fire and smoke
Log and ashes
Log and fire
Ashes and smoke
Tags
NGSS.MS-PS1-2
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
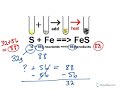
What is the total mass of the reactants in the first example?
28 kilograms
29 kilograms
31 kilograms
30 kilograms
Tags
NGSS.MS-PS1-5
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the second example, what is the mass of the unknown reactant if the total mass of the products is 88 grams and one reactant is 56 grams?
32 grams
56 grams
88 grams
44 grams
Tags
NGSS.MS-PS1-2
NGSS.MS-PS1-5
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total mass of the products in the second example?
56 grams
44 grams
88 grams
32 grams
Tags
NGSS.MS-PS1-5
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the third example, if the total mass of the reactants is 50 grams, what is the mass of the product?
20 grams
30 grams
40 grams
50 grams
Tags
NGSS.MS-PS1-5
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total mass of the reactants in the third example?
20 grams
30 grams
40 grams
50 grams
Tags
NGSS.MS-PS1-5
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the final example, what is the mass of the unknown product if the total mass of the reactants is 80 grams and one product is 44 grams?
36 grams
44 grams
80 grams
64 grams
Tags
NGSS.MS-PS1-5
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total mass of the reactants in the final example?
16 grams
64 grams
80 grams
44 grams
Tags
NGSS.MS-PS1-5
10.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the key principle of the conservation of mass demonstrated in all examples?
Mass of reactants is equal to mass of products.
Mass of reactants is less than mass of products.
Mass of products is always greater than mass of reactants.
Mass of reactants is always greater than mass of products.
Tags
NGSS.MS-PS1-5
Explore all questions with a free account
Similar Resources on Quizizz

11 questions
Law of Conservation of Mass Concepts
Interactive video
•
8th - 10th Grade

11 questions
Balancing Chemical Reactions and the Law of Conservation of Mass
Interactive video
•
6th - 10th Grade

11 questions
Mole Ratio and Stoichiometry Exam Review
Interactive video
•
6th - 10th Grade

11 questions
Exploring Endothermic and Exothermic Reactions
Interactive video
•
6th - 10th Grade

11 questions
Understanding Balanced Equations and Stoichiometry
Interactive video
•
7th - 10th Grade

11 questions
Mastering Stoichiometry Calculations with Mass
Interactive video
•
6th - 10th Grade

11 questions
Understanding the Law of Conservation of Mass
Interactive video
•
6th - 9th Grade

6 questions
Chemical Reactions
Interactive video
•
6th - 9th Grade
Popular Resources on Quizizz

17 questions
CAASPP Math Practice 3rd
Quiz
•
3rd Grade

20 questions
math review
Quiz
•
4th Grade

21 questions
6th Grade Math CAASPP Practice
Quiz
•
6th Grade

13 questions
Cinco de mayo
Interactive video
•
6th - 8th Grade

20 questions
Reading Comprehension
Quiz
•
5th Grade

20 questions
Types of Credit
Quiz
•
9th - 12th Grade

10 questions
4th Grade Math CAASPP (part 1)
Quiz
•
4th Grade

45 questions
5th Grade CAASPP Math Review
Quiz
•
5th Grade
Discover more resources for Science

20 questions
Evolution and Natural Selection
Quiz
•
7th Grade

10 questions
Exploring Newton's Laws of Motion
Interactive video
•
6th - 10th Grade

50 questions
Physical Science Milestones Review, Part 1
Quiz
•
6th - 8th Grade

40 questions
Physical Science SSA Review
Quiz
•
8th Grade

45 questions
7th Grade Life Science End Of The Year Final Review
Quiz
•
7th Grade

46 questions
8th Science STAAR Review
Quiz
•
8th Grade

20 questions
Waves and Wave Properties
Quiz
•
6th - 8th Grade

16 questions
Sexual vs Asexual Reproduction
Quiz
•
7th Grade