
formal charge
Authored by SYAMSUL Moe
Chemistry
University
NGSS covered
Used 11+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
20 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What is the correct Lewis Dot Structure for ammonia NH3
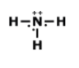
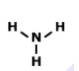
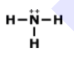
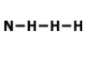
Tags
NGSS.HS-PS1-2
NGSS.HS-PS1-1
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

What is the formal charge for Oxygen
Tags
NGSS.HS-PS1-2
NGSS.HS-PS1-1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

What is the formal charge on the oxygen atom at the top of this Lewis dot structure?
0
-1
+1
-3
4.
MULTIPLE CHOICE QUESTION
30 sec • 5 pts

Based on formal charge, which Lewis structure best represents a sulfuric acid molecule?
Structure A is the better structure because all of its atoms have a formal charge of zero.
Structure A is the better structure because NOT all of its atoms have a formal charge of zero.
Structure B is the better structure because all of its atoms have a formal charge of zero.
Structure B is the better structure because NOT all of its atoms have a formal charge of zero.
5.
MULTIPLE CHOICE QUESTION
30 sec • 5 pts
The PF5 molecule exists, whereas NF5 does not. Which one of the following is the BEST explanation for this fact?
Phosphorus can use d orbitals in bonding, whereas nitrogen cannot.
The first five ionization energies of nitrogen are too high, but those of phosphorus are not.
Phosphorus has a core charge that must pull over a greater distance due to having a greater number of main energy levels.
Phosphorus has a lower electronegativity and is, therefore, able to hold a greater number of shared electrons in bonds.
Tags
NGSS.HS-PS1-2
NGSS.HS-PS1-1
6.
MULTIPLE CHOICE QUESTION
1 min • 5 pts
How many resonance structure for SO2 (without expanding the octet)?
1
2
3
4
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Sulfate ion can be drawn many ways. If you minimize formal charge on S, how many S=O should be drawn?
0
1
2
3
4
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

18 questions
CHEMICAL EQUILIBRIUM
Quiz
•
University

15 questions
Biossegurança Primeira Unidade
Quiz
•
University

16 questions
Chủ đề: Oxi-SO2-SO3-H2S
Quiz
•
10th Grade - Professi...

20 questions
CARBONYL COMPOUNDS
Quiz
•
10th Grade - University

20 questions
Acids and Bases
Quiz
•
University

15 questions
Simulacro Cup fac. salud humana part. 2
Quiz
•
University

18 questions
Revisão Farmacologia dos sistemas
Quiz
•
University

20 questions
Carbohydrates and Energy Metabolism
Quiz
•
University
Popular Resources on Wayground

8 questions
2 Step Word Problems
Quiz
•
KG - University

20 questions
Comparing Fractions
Quiz
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Latin Bases claus(clois,clos, clud, clus) and ped
Quiz
•
6th - 8th Grade

22 questions
fractions
Quiz
•
3rd Grade

7 questions
The Story of Books
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

8 questions
2 Step Word Problems
Quiz
•
KG - University

7 questions
Comparing Fractions
Interactive video
•
1st Grade - University

7 questions
Force and Motion
Interactive video
•
4th Grade - University

10 questions
14.2 Independent/Dependent Variables
Quiz
•
KG - University

18 questions
Great Lakes States
Quiz
•
KG - University

7 questions
DNA, Chromosomes, Genes, and Traits: An Intro to Heredity
Interactive video
•
11th Grade - University

7 questions
Reflexive Verbs in Spanish
Lesson
•
9th Grade - University

7 questions
Narrative Writing 1
Interactive video
•
4th Grade - University
