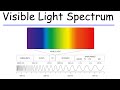
Which color of light has the longest wavelength?
Photon Energy and Light Properties
Interactive Video
•
Mia Campbell
•
Physics, Science
•
9th - 12th Grade
•
5 plays
•
Easy
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Green
Yellow
Blue
Red
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the wavelength of red light approximately?
300 nm
500 nm
690 nm
400 nm
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which color of light has the highest frequency?
Red
Yellow
Green
Blue
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula to calculate frequency from wavelength?
f = c / λ
f = c * λ
f = λ / c
f = λ * c
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the frequency of a photon with a wavelength of 460 nm?
5.00 x 10^14 Hz
6.52 x 10^14 Hz
3.00 x 10^8 Hz
1.00 x 10^12 Hz
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is Planck's constant value used in energy calculations?
6.626 x 10^-34 J·s
3.00 x 10^8 m/s
1.60 x 10^-19 J
9.81 m/s^2
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How much energy does a photon of blue light with a wavelength of 460 nm have in electron volts?
1.5 eV
2.7 eV
2.0 eV
3.0 eV
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the minimum voltage required to power a blue LED?
1.5 volts
2.7 volts
2.0 volts
3.5 volts
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the energy of a red photon with a wavelength of 625 nm in electron volts?
2.5 eV
1.5 eV
1.99 eV
3.0 eV
10.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the relationship between light frequency, wavelength, and energy?
Frequency is inversely proportional to energy
Energy is inversely proportional to frequency
Energy is directly proportional to frequency
Wavelength is directly proportional to energy
Explore all questions with a free account
Similar Resources on Quizizz

11 questions
Planck's Quantum Theory and Energy Flow in Atomic Models
Interactive video
•
9th - 12th Grade

11 questions
Photoelectric Effect and Photon Energy
Interactive video
•
9th - 12th Grade

6 questions
The Nature of Light: Video Quiz
Interactive video
•
10th - 12th Grade

6 questions
The Nature of Light: Video Quiz
Interactive video
•
10th - 12th Grade

11 questions
Understanding the Photoelectric Effect
Interactive video
•
9th - 12th Grade

11 questions
Electromagnetic Spectrum Dynamics and Photon Energy Relationships
Interactive video
•
9th - 12th Grade

11 questions
Energy Levels and Light Emission
Interactive video
•
9th - 12th Grade

11 questions
Photon Frequency and Wavelength Concepts
Interactive video
•
9th - 12th Grade
Popular Resources on Quizizz

17 questions
CAASPP Math Practice 3rd
Quiz
•
3rd Grade

20 questions
math review
Quiz
•
4th Grade

21 questions
6th Grade Math CAASPP Practice
Quiz
•
6th Grade

13 questions
Cinco de mayo
Interactive video
•
6th - 8th Grade

20 questions
Reading Comprehension
Quiz
•
5th Grade

20 questions
Types of Credit
Quiz
•
9th - 12th Grade

10 questions
4th Grade Math CAASPP (part 1)
Quiz
•
4th Grade

45 questions
5th Grade CAASPP Math Review
Quiz
•
5th Grade
Discover more resources for Physics

45 questions
Physics Semester 2 Review
Quiz
•
11th Grade

52 questions
AP Physics 1 Review
Quiz
•
11th Grade

50 questions
Physics semester 2 review
Quiz
•
11th Grade

40 questions
Light and EM Waves
Quiz
•
11th - 12th Grade

7 questions
EOY REVIEW 4
Lesson
•
10th - 12th Grade

82 questions
Physics Final Review
Quiz
•
11th Grade

20 questions
Electricity/Magnets
Quiz
•
9th Grade

40 questions
Physics Semester 1 Final Review
Quiz
•
9th - 12th Grade