Energy Levels and Light Emission
Interactive Video
•
Physics, Chemistry, Science
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of using gas spectra in atomic studies?
To identify the chemical composition of gases
To calculate the mass of atoms
To understand the internal structure of atoms
To measure the temperature of gases
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens when a gas is subjected to an electric discharge or heat?
It becomes solid
It changes color
It emits light
It absorbs light
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to Bohr, why don't electrons emit light continuously?
They are not charged
They are in specific orbits where they don't lose energy
They are stationary
They are too far from the nucleus
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
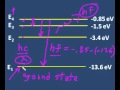
What is the ground state energy level for hydrogen according to Bohr?
-3.4 electron volts
13.6 electron volts
-13.6 electron volts
0 electron volts
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens when an electron moves from a higher energy level to a lower one?
It becomes neutral
It gains energy
It emits a photon
It absorbs a photon
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the energy difference between two levels related to the wavelength of light emitted?
Inversely proportional
Not related
Directly proportional
Equal to the wavelength
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the energy level of E2 in hydrogen?
-1.5 electron volts
0 electron volts
-3.4 electron volts
-13.6 electron volts
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Emission and Absorption Spectra Concepts
Interactive video
•
9th - 12th Grade

11 questions
Light and Spectrum Concepts
Interactive video
•
9th - 12th Grade

8 questions
Line Spectra: The Fingerprints of Atoms
Interactive video
•
10th Grade - University

8 questions
Line Spectra: The Fingerprints of Atoms
Interactive video
•
10th Grade - University

11 questions
Exploring The Electromagnetic Spectrum: Energy, Wavelength, And Frequency
Interactive video
•
9th - 12th Grade

11 questions
Quantum Mechanics and Electron Configuration
Interactive video
•
9th - 12th Grade

11 questions
Photoelectric Effect and Light Wavelengths
Interactive video
•
10th - 12th Grade

11 questions
Electromagnetic Spectrum Dynamics and Photon Energy Relationships
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

11 questions
Hallway & Bathroom Expectations
Quiz
•
6th - 8th Grade

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade
Discover more resources for Physics

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

40 questions
LSHS Student Handbook Review: Pages 7-9
Quiz
•
11th Grade

24 questions
Scientific method and variables review
Quiz
•
9th Grade

10 questions
Characteristics of Life
Quiz
•
9th - 10th Grade

19 questions
Mental Health Vocabulary Pre-test
Quiz
•
9th Grade