
Molecular Geometry and Polarity
Authored by Charles Martinez
Science
9th - 12th Grade
NGSS covered

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
25 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which is the correct molecular structure for carbon dioxide (CO2)?




2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What is the correct Lewis Dot Structure for ammonia NH3?
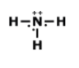
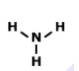
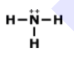
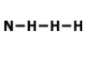
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to VSEPR, molecules adjust their shapes to keep which of the following as far away as possible?
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to the octet rule most elements need _______ valence electrons.
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Hydrogen needs _____ electrons in its valence shell to be stable.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Metals and nonmetals form which kind of bond?
Tags
NGSS.HS-PS1-1
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How strongly an atom attracts electrons towards itself in a bond is called...
Ionization energy
Electronegativity
Electricity
Nuclear force
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

20 questions
Ecology Notes RECAP Quiz
Quiz
•
12th Grade

20 questions
Test: Biotechnology
Quiz
•
11th Grade

20 questions
Origin of Life and Evidence of Evolution
Quiz
•
9th - 12th Grade

20 questions
Geological and Hydrometeorological Hazards
Quiz
•
12th Grade
![Topic A1, A2, A3 Revision Quiz [20 marks]](https://cf.quizizz.com/image/image-loader.svg)
20 questions
Topic A1, A2, A3 Revision Quiz [20 marks]
Quiz
•
11th Grade

20 questions
2024 Mid Term Final Review
Quiz
•
9th Grade

20 questions
Astronomy 1
Quiz
•
9th - 12th Grade

20 questions
Gemstones - ELL Science
Quiz
•
9th - 12th Grade
Popular Resources on Wayground

8 questions
Spartan Way - Classroom Responsible
Quiz
•
9th - 12th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

14 questions
Boundaries & Healthy Relationships
Lesson
•
6th - 8th Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

3 questions
Integrity and Your Health
Lesson
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

9 questions
FOREST Perception
Lesson
•
KG

20 questions
Main Idea and Details
Quiz
•
5th Grade
Discover more resources for Science

25 questions
Naming Ionic and Covalent Compounds
Quiz
•
9th Grade

10 questions
Exploring the Evolution of the Peppered Moth
Interactive video
•
6th - 10th Grade

35 questions
DNA Structure and Replication
Quiz
•
10th Grade

10 questions
Exploring Trophic Levels and Food Pyramids
Interactive video
•
6th - 10th Grade

28 questions
Spring DPM Review
Quiz
•
9th Grade

15 questions
Chemical Reactions (Types of Chemical Reactions)
Interactive video
•
11th Grade

10 questions
Symbiotic Relationships
Lesson
•
9th - 12th Grade

13 questions
DNA Mutations
Quiz
•
9th Grade

