
Quantum Mechanical Model of the Atom
Authored by Lisa Thompson
Science
9th Grade
NGSS covered
Used 3+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
25 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
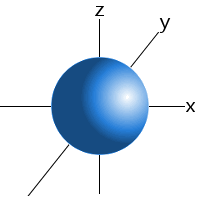
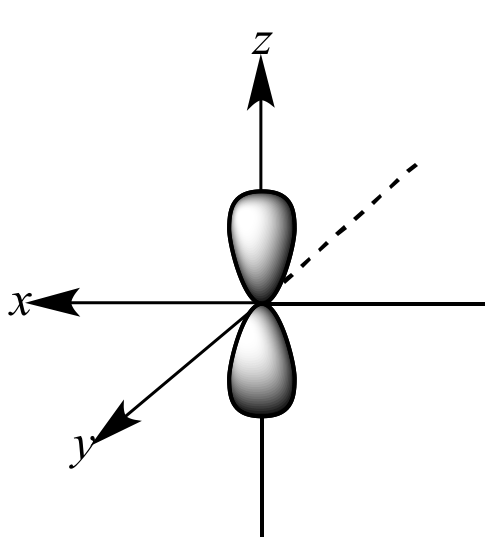
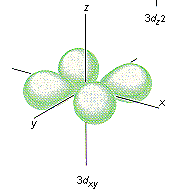
Which shape represents the shape of an "s" orbital?
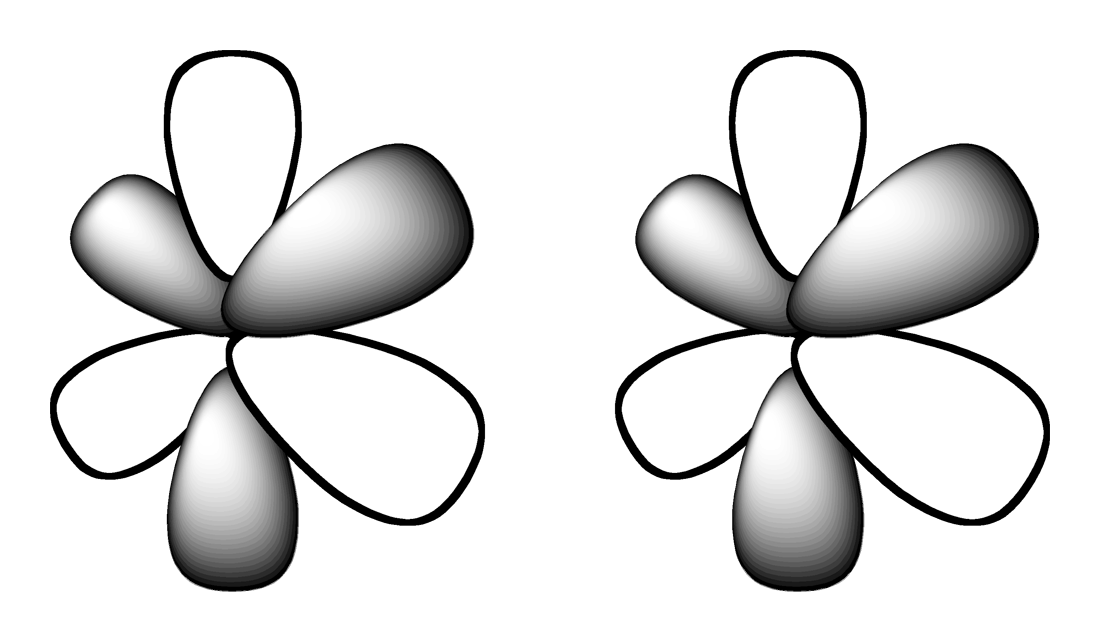
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which shape represents the shape of an "p" orbital?



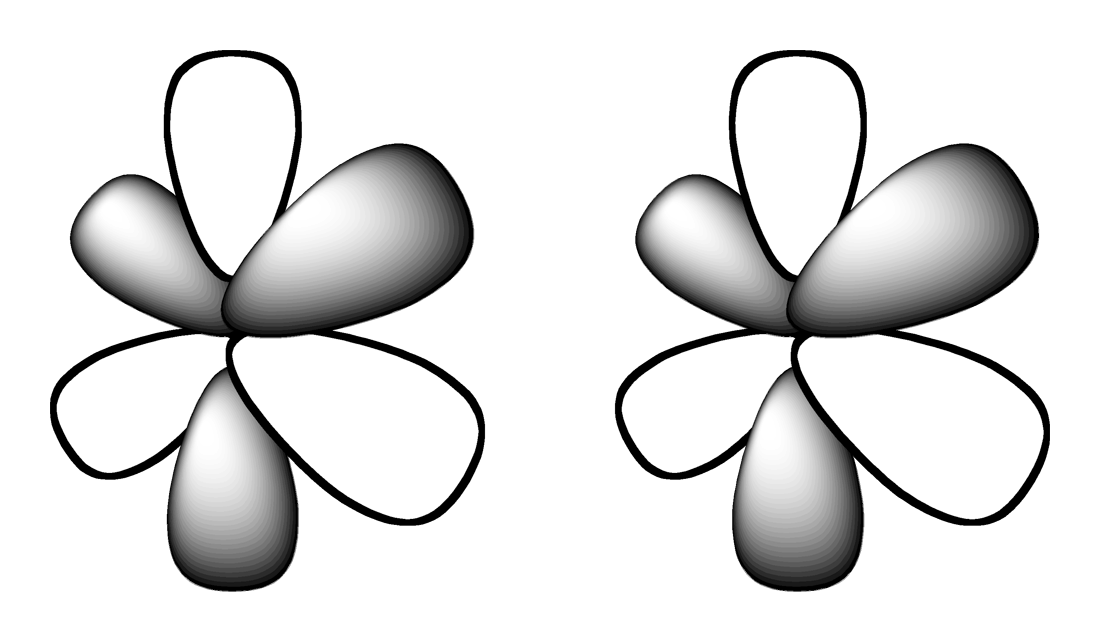
3.
OPEN ENDED QUESTION
1 min • 1 pt

Compare and contrast the Bohr Model and the Quantum Model of the atom. Describe at least two key similarities and at least one key difference.
Evaluate responses using AI:
OFF
4.
MULTIPLE SELECT QUESTION
1 min • 1 pt
There are four different orbital shapes. Select them from the following list.
"s"
"f"
"g"
"d"
"p"
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
In the quantum mechanical model of the atom, where are electrons most likely to be found?
Moving in fixed orbits around the nucleus
Embedded within the nucleus
In specific regions called electron clouds or orbitals
Uniformly distributed around the nucleus
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
In the quantum-mechanical model of the atom, an orbital is defined as a
region of the most probable proton location.
region of the most probable electron location.
circular path traveled by an electron around an orbital.
circular path traveled by a proton around an orbital.
Answer explanation
In the quantum-mechanical model of the atom, an orbital is defined as a region of the most probable electron location.
7.
MULTIPLE SELECT QUESTION
1 min • 1 pt
What is the fundamental difference between the Bohr model of an atom and the quantum mechanical model? Select all that apply.
Bohr model considers electron to be a particle; quantum mechanics considers it to be a wave.
Bohr model describes electron with 1 quantum number; quantum model uses 4 quantum.
Bohr model describes electrons in orbits; quantum model describes electrons in orbitals.
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

21 questions
9.1 Quiz: rotation and revolution
Quiz
•
8th - 11th Grade

21 questions
What is current?
Quiz
•
9th Grade

22 questions
Functions in RobotC
Quiz
•
7th - 11th Grade

23 questions
Covalent bonding
Quiz
•
9th - 11th Grade

25 questions
Week 28 - Chapter 20 Sound
Quiz
•
7th - 10th Grade

25 questions
Habitats and Food Chains Quiz
Quiz
•
5th Grade - University

25 questions
Blood Vessels
Quiz
•
7th Grade - University

25 questions
Potential and Kinetic Energy Transformation
Quiz
•
8th Grade - University
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

10 questions
Probability Practice
Quiz
•
4th Grade

15 questions
Probability on Number LIne
Quiz
•
4th Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

6 questions
Appropriate Chromebook Usage
Lesson
•
7th Grade

10 questions
Greek Bases tele and phon
Quiz
•
6th - 8th Grade
Discover more resources for Science

25 questions
Naming Ionic and Covalent Compounds
Quiz
•
9th Grade

10 questions
Exploring Weathering, Erosion, and Deposition Processes
Interactive video
•
6th - 10th Grade

26 questions
Unit 8b Review
Quiz
•
9th Grade

14 questions
Natural Selection and Adaptation
Lesson
•
9th - 12th Grade

10 questions
Exploring Water Pollution and Conservation
Interactive video
•
6th - 10th Grade

10 questions
Exploring the Human Respiratory System
Interactive video
•
6th - 10th Grade

30 questions
Unit 2C Progress Check (Biosphere 3)
Quiz
•
9th - 12th Grade

10 questions
Exploring Light and Waves Concepts
Interactive video
•
6th - 10th Grade