
Timberlake Chemistry
Quiz
•
Science
•
8th Grade
•
Practice Problem
•
Hard
+4
Standards-aligned

Lisa Thompson
FREE Resource
Enhance your content in a minute
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

How much liquid water can you change 20 C if you apply 20,000 J?
500 g
100,000 g
250 g
350 g
Tags
NGSS.MS-PS1-4
NGSS.MS-PS3-4
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
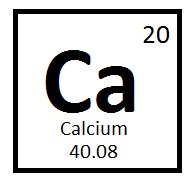
What is the atomic mass of this element?
20
60
40
41
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

What does a represent?
Proton
Neutron
Electron
Tags
NGSS.MS-PS1-1
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

What is the atomic number of this element?
20
60
40
41
Tags
NGSS.MS-PS1-1
5.
LABELLING QUESTION
1 min • 1 pt
Identify which are the products and reactants
reactants
products
Tags
NGSS.MS-PS1-2
NGSS.MS-PS1-5
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
The Law of Conservation of Mass states that
compounds and elements are the same
elements can be destroyed
matter cannot be created or destroyed, but can be transformed
mass is the same for all the objects if they need to exist
Tags
NGSS.MS-PS1-5
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
How is a chemical change different from a physical change?
there is no difference
a physical change creates a new substance
a chemical change creates a new substance
Tags
NGSS.MS-PS1-5
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

13 questions
Atomic Structure
Quiz
•
7th Grade - University

13 questions
Minerals
Quiz
•
8th Grade

10 questions
Abeka Science 8th grade Quiz 17
Quiz
•
8th Grade

13 questions
Quiz on Waves and Sunscreen
Quiz
•
8th Grade - University

10 questions
Grade 8 Science - Mr. Randy.
Quiz
•
6th - 8th Grade

15 questions
Y9 chemistry quiz on air and water
Quiz
•
7th - 10th Grade

15 questions
Science 8 Q1 M4: Effect of Temperature to the Speed of Sound
Quiz
•
8th Grade

10 questions
Our Future
Quiz
•
KG - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Science

20 questions
Cell Organelles and Functions
Quiz
•
6th - 8th Grade

33 questions
Grade 6 Quarter 3 PMA 5 Review
Quiz
•
6th - 8th Grade

10 questions
Exploring the Rock Cycle: Types and Formation
Interactive video
•
6th - 8th Grade

10 questions
Exploring the Layers of the Earth
Interactive video
•
6th - 10th Grade

14 questions
Biodiversity and Sustainability Quiz
Quiz
•
8th Grade

20 questions
Heredity Punnett square review
Quiz
•
8th Grade

23 questions
Newton's 3 Laws of Motion
Quiz
•
8th Grade

10 questions
Exploring the Rock Cycle
Interactive video
•
6th - 8th Grade
