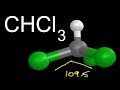
Molecular Geometry of CHCl3
Interactive Video
•
Amelia Wright
•
Chemistry
•
9th - 10th Grade
•
Hard
00:00
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE
30 sec • 1 pt
What is the purpose of using Lewis structures in determining molecular geometry?
2.
MULTIPLE CHOICE
30 sec • 1 pt
In the AXN notation for CHCl3, what does 'X' represent?
3.
MULTIPLE CHOICE
30 sec • 1 pt
How many atoms are bonded to the central carbon in CHCl3?
4.
MULTIPLE CHOICE
30 sec • 1 pt
What is the molecular geometry of CHCl3 according to the AX4 notation?
5.
MULTIPLE CHOICE
30 sec • 1 pt
What is the bond angle in a tetrahedral molecular geometry?
6.
MULTIPLE CHOICE
30 sec • 1 pt
In the 3D visualization of CHCl3, which atom is represented by the color green?
7.
MULTIPLE CHOICE
30 sec • 1 pt
Where is the hydrogen atom located in the 3D structure of CHCl3?
8.
MULTIPLE CHOICE
30 sec • 1 pt
What is the significance of the bond angles in CHCl3's molecular geometry?
9.
MULTIPLE CHOICE
30 sec • 1 pt
Which theory explains the arrangement of atoms in CHCl3?
10.
MULTIPLE CHOICE
30 sec • 1 pt
What is the overall shape of CHCl3 as discussed in the video?
Explore all questions with a free account
Similar Resources on Quizizz

8 questions
SF2 Molecular Geometry and Polarity
•
9th - 10th Grade

8 questions
Methane and Alkane Properties
•
9th - 10th Grade

8 questions
Molecular Geometry of NH3
•
9th - 10th Grade

8 questions
Molecular Geometry of CO2
•
9th - 10th Grade

11 questions
Molecular Geometry of H2SO4
•
9th - 10th Grade

10 questions
Molecular Geometry of NCl3
•
9th - 10th Grade

9 questions
Molecular Geometry of N2O
•
9th - 10th Grade

9 questions
Polarity and Geometry of BCl3
•
9th - 10th Grade
Popular Resources on Quizizz

17 questions
CAASPP Math Practice 3rd
•
3rd Grade

15 questions
Grade 3 Simulation Assessment 1
•
3rd Grade

37 questions
Math STAAR Review
•
4th Grade

12 questions
Earth Day
•
4th Grade

19 questions
HCS Grade 5 Simulation Assessment_1 2425sy
•
5th Grade

20 questions
Science STAAR Review! 23-24
•
5th Grade

22 questions
HCS Grade 4 Simulation Assessment_1 2425sy
•
4th Grade

16 questions
Grade 3 Simulation Assessment 2
•
3rd Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
•
9th - 12th Grade

20 questions
Types of Chemical Reactions
•
10th Grade

21 questions
Kinetic Theory of Gases and Pressure Conversions Review
•
9th - 12th Grade

18 questions
Naming Acids and Bases
•
10th Grade

21 questions
Collision theory and reaction rates
•
10th - 11th Grade

20 questions
Energy Transformations
•
9th - 12th Grade

24 questions
Types of Chemical Reactions
•
10th Grade

52 questions
Unit 8: Gas Laws Review
•
10th Grade