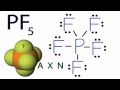
Molecular Geometry of PF5
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in determining the molecular geometry of PF5?
Measuring bond angles
Using the AXN notation
Counting non-bonding electron pairs
Looking at the Lewis structure
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to VSEPR theory, how do fluorine atoms arrange themselves around the phosphorus atom in PF5?
In a square planar arrangement
In a linear arrangement
As close to each other as possible
As far apart as possible while remaining bonded
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of PF5 as determined by the AXN notation?
Trigonal bipyramidal
Tetrahedral
Trigonal planar
Octahedral
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the AXN notation for PF5, what does 'X' represent?
The total number of electron pairs
The number of atoms bonded to the central atom
The number of non-bonding electron pairs
The central atom
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle between the equatorial fluorine atoms in PF5?
180°
120°
90°
109.5°
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle between the axial and equatorial fluorine atoms in PF5?
109.5°
180°
120°
90°
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is NOT a feature of the trigonal bipyramidal geometry?
Five bonded atoms
Two different bond angles
A central atom with non-bonding pairs
Equatorial and axial positions
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

7 questions
Shops and Shopping in the UK and the USA
Interactive video
•
8th Grade

6 questions
Circles Quiz
Interactive video
•
10th - 12th Grade

11 questions
Angle Relationships in Circles
Interactive video
•
9th - 12th Grade

8 questions
An introduction into the law of sines
Interactive video
•
9th - 10th Grade

6 questions
Solving Cone Volume Word Problems
Interactive video
•
9th - 10th Grade

11 questions
Tangents and Chords in Parabolas
Interactive video
•
9th - 10th Grade

11 questions
Centroid and Circle Relationships
Interactive video
•
9th - 10th Grade

11 questions
Equilateral Triangle and Circle Areas
Interactive video
•
8th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade