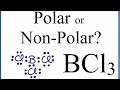
Polarity and Geometry of BCl3
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in determining if BCl3 is polar or nonpolar?
Measure the bond angles
Draw the Lewis structure
Check the molecular weight
Count the number of atoms
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What theory is used to predict the 3D arrangement of atoms in BCl3?
Crystal Field Theory
VSEPR Theory
Molecular Orbital Theory
Hybridization Theory
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many chlorine atoms are bonded to the central boron atom in BCl3?
Five
Four
Three
Two
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of BCl3?
Tetrahedral
Bent
Linear
Trigonal Planar
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the chlorine atoms in BCl3 according to VSEPR theory?
They attract each other
They repel each other
They cluster together
They form a linear shape
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does BCl3 have no net dipole moment?
It is asymmetrical
It has polar bonds
It has lone pairs
It is symmetrical
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of a molecule being symmetrical?
It has a net dipole
It is always polar
It has no net dipole
It is always nonpolar
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following best describes the polarity of BCl3?
Polar due to asymmetry
Nonpolar due to symmetry
Polar due to lone pairs
Nonpolar due to polar bonds
Similar Resources on Wayground

8 questions
Balancing Chemical Equations Practice
Interactive video
•
9th - 10th Grade

9 questions
Balancing Chemical Reactions
Interactive video
•
9th - 10th Grade

9 questions
Fluoride Ion Properties and Structure
Interactive video
•
9th - 10th Grade

6 questions
Manganese and Oxygen Compounds
Interactive video
•
9th - 10th Grade

6 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

7 questions
Molar Mass Calculations and Concepts
Interactive video
•
9th - 10th Grade

8 questions
Molecular Geometry and Iodine
Interactive video
•
9th - 10th Grade

8 questions
Molecular Formula and Molar Mass of Acidic Anhydride
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

20 questions
MINERS Core Values Quiz
Quiz
•
8th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade

10 questions
How to Email your Teacher
Quiz
•
Professional Development

15 questions
Order of Operations
Quiz
•
5th Grade
Discover more resources for Chemistry

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

19 questions
Lewis Dot Structures -Review and Master
Quiz
•
10th Grade

10 questions
Electron Configuration, Orbital Notation, & Dot diagrams
Lesson
•
9th - 12th Grade

10 questions
Intro to Atoms Vocabulary Quiz
Quiz
•
8th - 10th Grade

20 questions
Naming Polyatomic Ionic compounds
Quiz
•
9th - 12th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade