What are the two types of atoms attached to the carbon atom on the left in acetylene?
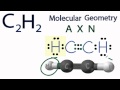
Acetylene Molecular Geometry and Bonding
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Carbon and Oxygen
Hydrogen and Carbon
Hydrogen and Nitrogen
Hydrogen and Oxygen
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of acetylene?
Tetrahedral
Trigonal Planar
Linear
Bent
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do the hydrogen and carbon atoms arrange themselves in acetylene?
In a triangular shape
In a straight line
In a circular pattern
In a zigzag pattern
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of bond is present between the carbon atoms in acetylene?
Triple bond
Quadruple bond
Single bond
Double bond
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In AXN notation, what does 'X' represent for acetylene?
Number of hydrogen atoms
Number of electron pairs
Number of atoms bonded to the central atom
Number of lone pairs
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the value of 'X' in the AXN notation for acetylene?
4
2
1
3
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle in a linear molecular geometry like acetylene?
360°
180°
120°
90°
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a bond angle of 180° indicate about the shape of a molecule?
It forms a circle
It forms a straight line
It forms a square
It forms a triangle
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which notation can be used to determine the molecular geometry of acetylene?
Molecular Orbital Theory
AXN Notation
Lewis Structure
VSEPR Theory
Popular Resources on Quizizz

10 questions
Chains by Laurie Halse Anderson Chapters 1-3 Quiz
Quiz
•
6th Grade

20 questions
math review
Quiz
•
4th Grade

15 questions
Character Analysis
Quiz
•
4th Grade

12 questions
Multiplying Fractions
Quiz
•
6th Grade

30 questions
Biology Regents Review #1
Quiz
•
9th Grade

20 questions
Reading Comprehension
Quiz
•
5th Grade

20 questions
Types of Credit
Quiz
•
9th - 12th Grade

50 questions
Biology Regents Review: Structure & Function
Quiz
•
9th - 12th Grade