Exploring the Bohr Model and Emission Spectra
Interactive Video
•
Science
•
6th - 10th Grade
•
Practice Problem
•
Medium
Standards-aligned
Sophia Harris
Used 6+ times
FREE Resource
Standards-aligned
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the range of wavelengths in the electromagnetic spectrum?
From greater than 10 meters to less than a millionth of a meter
From greater than 1000 meters to less than a trillionth of a meter
From greater than 1 meter to less than a billionth of a meter
From greater than 100 meters to less than a trillionth of a meter
Tags
NGSS.HS-PS4-1
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is NOT a type of electromagnetic wave?
Sound waves
Microwaves
Gamma rays
Radio waves
Tags
NGSS.HS-PS4-3
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What color does hydrogen emit when energized with an electric current?
Blue
Red
Pale pinkish
Green
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which color corresponds to a wavelength of 486 nanometers in hydrogen's emission spectrum?
Violet
Blue-green
Blue
Red
Tags
NGSS.HS-PS4-1
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
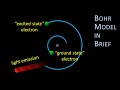
In Bohr's atomic model, what happens when an electron transitions from a higher to a lower energy level?
It remains in the same orbit
It absorbs light
It emits light
It disappears
Tags
NGSS.HS-PS4-3
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the term for the lowest energy state of an electron?
Excited state
Intermediate state
Ground state
Neutral state
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the energy change of an electron from n=3 to n=2 in hydrogen's emission spectrum?
Green light
Red light
Blue light
Violet light
Tags
NGSS.HS-PS4-3
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

8 questions
2 Step Word Problems
Quiz
•
KG - University

20 questions
Comparing Fractions
Quiz
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Latin Bases claus(clois,clos, clud, clus) and ped
Quiz
•
6th - 8th Grade

22 questions
fractions
Quiz
•
3rd Grade

7 questions
The Story of Books
Quiz
•
6th - 8th Grade
Discover more resources for Science

12 questions
Newton's Laws of Motion
Lesson
•
6th - 8th Grade

20 questions
Elements, Compounds and Mixtures
Quiz
•
8th Grade

10 questions
Exploring Earth's Seasons and Their Causes
Interactive video
•
6th - 8th Grade

22 questions
Wave Properties
Lesson
•
7th Grade

8 questions
VP #9: Amoeba Sisters Pedigrees
Interactive video
•
7th Grade

20 questions
Cells! Cell Theory and Characteristics of Eukaryotes/Prokaryotes
Quiz
•
6th Grade

11 questions
The Water Cycle
Quiz
•
6th Grade

20 questions
Natural Selection and Adaptations
Quiz
•
6th - 8th Grade