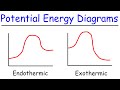
Exploring Endothermic and Exothermic Reactions
Interactive Video
•
Chemistry
•
6th - 10th Grade
•
Hard
Amelia Wright
Used 1+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the sign of enthalpy change in an endothermic reaction?
Positive
Negative
Zero
Cannot be determined
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In an exothermic reaction, the energy of the reactants is:
Unrelated to the energy of the products
Greater than the energy of the products
Less than the energy of the products
Equal to the energy of the products
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the y-axis represent in a potential energy diagram?
Activation energy
Potential energy of the system
Reaction coordinate
Enthalpy change
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
For an endothermic reaction, the potential energy of the products compared to the reactants is:
The same
Lower
Variable
Higher
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What effect does a catalyst have on a reaction?
Increases the energy of reactants
Decreases the energy of products
Lowers the activation energy
Increases the enthalpy change
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which step is considered the slow step in a multi-step reaction?
The step with the lowest activation energy
The step with the highest activation energy
The last step
The first step
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Is melting ice an endothermic or exothermic process?
Endothermic
Exothermic
Adiabatic
Isothermic
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Exploring Endothermic and Exothermic Reactions
Interactive video
•
6th - 10th Grade

11 questions
Exploring Energy Changes in Chemical Reactions
Interactive video
•
6th - 10th Grade

11 questions
Key Concepts in Thermodynamics: Bond Enthalpy and Equations
Interactive video
•
6th - 10th Grade

11 questions
Exploring the Forces Behind Chemical Reactions
Interactive video
•
6th - 10th Grade

11 questions
Endothermic and Exothermic Reactions
Interactive video
•
6th - 10th Grade

11 questions
Exploring Heat and Enthalpy in Thermochemistry
Interactive video
•
6th - 10th Grade

9 questions
Exploring Endothermic and Exothermic Reactions
Interactive video
•
6th - 10th Grade

8 questions
Soap-Making Process and Chemistry
Interactive video
•
6th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade