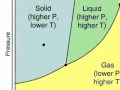
Exploring Phase Diagrams and States of Matter
Interactive Video
•
Science
•
6th - 8th Grade
•
Easy
Standards-aligned
Aiden Montgomery
Used 1+ times
FREE Resource
Standards-aligned
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a phase diagram typically illustrate about a substance?
Chemical composition
Physical state based on temperature and pressure
Biological properties
Electrical conductivity
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which phase is characterized by particles that are tightly packed but not fixed, allowing them to jiggle in place?
Liquid
Solid
Gas
Plasma
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the typical behavior of particles in a gas phase as observed in a phase diagram?
Fixed in a lattice structure
None of the above
Far apart and colliding occasionally
Closely packed and vibrating
Tags
NGSS.HS-PS2-1
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What distinguishes the liquid phase from the solid phase in terms of particle movement?
Particles are immobile
Particles are less restricted and move freely
Particles vibrate but do not move
Particles are tightly packed and static
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
At the solid-liquid line of a phase diagram, what is occurring with the particles?
Sublimation
Only freezing
Only melting
Both melting and freezing at equal rates
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
During sublimation as depicted in a phase diagram, particles transition from which phase to which?
Gas to solid
Gas to liquid
Solid to gas
Liquid to solid
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What occurs along the liquid-gas line in a phase diagram?
Only vaporization
Only condensation
Both vaporization and condensation at equal rates
Deposition and sublimation
Create a free account and access millions of resources
Similar Resources on Wayground

8 questions
Molecular Movement and Phase Changes in Butter and Olive Oil
Interactive video
•
6th - 8th Grade

11 questions
Phases Of Matter And Energy Changes In Chemistry And Physics
Interactive video
•
6th - 8th Grade

8 questions
Phases of Matter
Interactive video
•
6th - 9th Grade

11 questions
Phases Of Matter And Their Energy Dynamics
Interactive video
•
6th - 8th Grade

9 questions
States of Matter and Phase Changes
Interactive video
•
6th - 7th Grade

11 questions
Phase Change Diagrams: Analyzing States of Matter and Energy Transformations
Interactive video
•
6th - 8th Grade

11 questions
Phase Changes and Heating Curves
Interactive video
•
6th - 8th Grade

11 questions
Phases Of Water Energy And Temperature Dynamics
Interactive video
•
6th - 8th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Science

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

24 questions
Flinn Lab Safety Quiz
Quiz
•
5th - 8th Grade

20 questions
Lab Safety
Quiz
•
7th Grade

22 questions
Scientific Method and Variables
Quiz
•
8th Grade

20 questions
Scientific method and variables
Quiz
•
8th Grade

20 questions
Scientific Method
Quiz
•
7th Grade

20 questions
disney movies
Quiz
•
6th Grade

10 questions
Scientific Method and Variables
Quiz
•
6th Grade