Cycloalkanes and Their Properties
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Practice Problem
•
Hard
Sophia Harris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
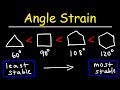
Which cycloalkane is the most stable?
Cyclopentane
Cyclohexane
Cyclobutane
Cyclopropane
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the least stable cycloalkane?
Cyclopropane
Cyclohexane
Cyclobutane
Cyclopentane
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which cycloalkane has the highest heat of combustion per CH2 group?
Cyclopropane
Cyclobutane
Cyclopentane
Cyclohexane
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why can cyclopropane undergo addition reactions with hydrogen gas?
It is a straight chain alkane
It has high ring strain
It is highly stable
It has low energy
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular formula of propane formed from cyclopropane?
C4H8
C3H6
C3H8
C4H10
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the ideal bond angle for an sp3 carbon?
108 degrees
90 degrees
60 degrees
109.5 degrees
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the interior angle of a cyclopropane ring?
108 degrees
90 degrees
120 degrees
60 degrees
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Student Life and Budget Management
Interactive video
•
10th - 12th Grade

10 questions
Friedel Crafts Reactions and Directing Effects
Interactive video
•
11th - 12th Grade

8 questions
Aromatics and Cyclic Compounds - Crash Course Chemistry
Interactive video
•
11th Grade - University

6 questions
What's the Best Position to Sleep In
Interactive video
•
11th Grade - University

8 questions
What are Social Roles?
Interactive video
•
10th - 12th Grade

8 questions
Music History - Stars And Stripes Forever
Interactive video
•
10th - 12th Grade

11 questions
Bill Nye and the Origins of Life
Interactive video
•
10th Grade - University

6 questions
CLEAN: Oxford battles Britain's historic floods
Interactive video
•
10th Grade - University
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade
Discover more resources for Chemistry

20 questions
Ionic Compound Nomenclature
Quiz
•
9th - 12th Grade

30 questions
ERHS Chem - Chapter 6 Covalent Compounds
Quiz
•
11th Grade

42 questions
Chemistry Final Exam Review
Quiz
•
9th - 12th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

35 questions
Chemistry Semester A Final Review
Quiz
•
11th Grade