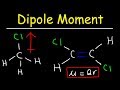
Understanding Dipole Moments and Ionic Character
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Practice Problem
•
Hard
Jackson Turner
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which factor does NOT affect the dipole moment of a bond?
Molecular weight
Bond length
Electronegativity difference
Atomic size
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is methyl chloride considered a polar molecule?
It has a symmetrical structure.
The dipole moments do not cancel out.
It has a net dipole moment of zero.
It contains only non-polar bonds.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the net dipole moment of carbon tetrachloride?
Zero
Less than zero
Greater than zero
Equal to the dipole moment of one C-Cl bond
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the context of dipole moments, what does the term 'vector sum' refer to?
The sum of all bond lengths
The total number of bonds in a molecule
The combined effect of all dipole moments in a molecule
The difference in electronegativity between two atoms
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which isomer has a net dipole moment, trans or cis?
Both isomers
Trans isomer
Cis isomer
Neither isomer
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in calculating the percent ionic character of a bond?
Determine the bond length
Calculate the experimental dipole moment
Find the dipole moment if the bond were 100% ionic
Measure the electronegativity difference
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the percent ionic character of a CO bond with an experimental dipole moment of 7 D?
32.6%
50%
20.8%
10.4%
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Fritz Haber and the Impact of Nitrogen Fixation
Interactive video
•
9th - 12th Grade

11 questions
Understanding Time, Traditions, and the Cosmos
Interactive video
•
9th - 12th Grade

11 questions
Chemistry Concepts and Properties
Interactive video
•
9th - 12th Grade

8 questions
7 enigmas de la ciencia
Interactive video
•
10th - 12th Grade

11 questions
CRISPR-Cas9 System Applications and Mechanisms
Interactive video
•
9th - 12th Grade

11 questions
Understanding Quarks and Particle Physics
Interactive video
•
9th - 12th Grade

11 questions
Understanding Quantum Chemistry and Molecular Bonding
Interactive video
•
9th - 12th Grade

11 questions
Semiconductor Concepts and Properties
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade
Discover more resources for Chemistry

20 questions
Ionic Compound Nomenclature
Quiz
•
9th - 12th Grade

30 questions
ERHS Chem - Chapter 6 Covalent Compounds
Quiz
•
11th Grade

42 questions
Chemistry Final Exam Review
Quiz
•
9th - 12th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

35 questions
Chemistry Semester A Final Review
Quiz
•
11th Grade