Understanding Calculate Structures
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Practice Problem
•
Hard
Lucas Foster
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a calculate structure similar to, but with lone pairs usually omitted?
Lewis structure
Structural formula
Empirical formula
Molecular formula
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
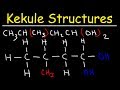
In the calculate structure of CH3CH2CH2CH2OH, how many hydrogen atoms are attached to the first carbon?
Four
One
Two
Three
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the example CH3CHBrCH2C(CH3)3, what is attached to the second carbon?
Three methyl groups
Oxygen
Two hydrogens
Hydrogen and bromine
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where are CH3 groups typically located in a calculate structure?
Attached to oxygen
In the middle of the chain
Next to bromine
At the end of the chain
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the example CH3CH(CH3)CH2CHOH, why can't a CH3 group be placed in the middle of the chain?
It is not chemically stable
It is not possible to draw
It would break the chain
It would result in a carbon with five bonds
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What should be done with a CH3 group that appears in the middle of a chain?
Place it outside the chain
Remove it
Attach it to the next carbon
Convert it to CH2
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the structure CH3CH2C(CH3)2CH2CHClCH2CF3, what is the functional group attached to the last carbon?
Chlorine
Oxygen
Bromine
Fluorine
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Stoichiometry Concepts
Interactive video
•
6th - 10th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Practice: E-Con, Orbital Notation, Noble Gas Notation
Quiz
•
10th Grade

20 questions
Covalent Bonding
Quiz
•
10th Grade

10 questions
Periodic Table Families and Groups
Quiz
•
10th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade

22 questions
Solubility Curve Practice
Quiz
•
10th Grade