Gas Laws and Mole Fractions
Interactive Video
•
Chemistry, Physics, Science
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does Dalton's Law of Partial Pressure state?
The total pressure of a gas mixture is the sum of the partial pressures of its components.
The total pressure of a gas mixture is the difference between the partial pressures of its components.
The total pressure of a gas mixture is the average of the partial pressures of its components.
The total pressure of a gas mixture is the product of the partial pressures of its components.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the example of oxygen collected over water, what is the vapor pressure of water at 24 degrees Celsius?
745 Torr
24.38 Torr
721 Torr
50 Atmospheres
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate the partial pressure of a gas using Dalton's Law?
By dividing the total pressure by the vapor pressure of water.
By adding the total pressure to the vapor pressure of water.
By subtracting the vapor pressure of water from the total pressure.
By multiplying the total pressure by the vapor pressure of water.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
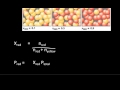
What is the mole fraction of a gas in a mixture?
The ratio of the gas's mass to the total mass of the mixture.
The ratio of the gas's volume to the total volume of the mixture.
The ratio of the gas's moles to the total moles of the mixture.
The ratio of the gas's pressure to the total pressure of the mixture.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the example with neon and oxygen, what is the total pressure in the gas tanks?
50 Atmospheres
721 Torr
24.38 Torr
745 Torr
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you convert mass to moles for calculating mole fraction?
By dividing the mass by the molar mass.
By multiplying the mass by the molar mass.
By adding the mass to the molar mass.
By subtracting the mass from the molar mass.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the mole fraction of oxygen in the mixture of neon and oxygen?
0.20999
0.5
1.0
0.9
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Gas Behavior and Dalton's Law of Partial Pressures Explained
Interactive video
•
9th - 12th Grade

11 questions
Mastering Dalton's Law and Partial Pressures in Gaseous Mixtures
Interactive video
•
9th - 12th Grade

11 questions
Gas Laws and Partial Pressures
Interactive video
•
9th - 12th Grade

11 questions
Mole Fractions and Gas Laws
Interactive video
•
10th - 12th Grade

11 questions
Gas Mixture Calculations
Interactive video
•
9th - 12th Grade

11 questions
Mole Fraction and Partial Pressure Concepts
Interactive video
•
9th - 12th Grade

11 questions
Gas Laws and Partial Pressures
Interactive video
•
9th - 12th Grade

6 questions
Lechatliers principle and pressure effect
Interactive video
•
10th Grade - University
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade