Properties and Bonds of Water
Interactive Video
•
Chemistry, Science, Biology
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the composition of a water molecule?
Two hydrogen atoms and two oxygen atoms
Two hydrogen atoms and one oxygen atom
Two oxygen atoms and one hydrogen atom
One hydrogen atom and one oxygen atom
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does the oxygen atom in a water molecule have a partial negative charge?
Because it shares electrons equally
Because it is more electronegative
Because it has more protons
Because it is less electronegative
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a unique feature of hydrogen bonds in water?
They form between polar molecules
They are stronger than covalent bonds
They occur only in water
They are a type of ionic bond
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do hydrogen bonds affect the properties of water compared to methane?
They make methane form hydrogen bonds
They make water non-polar
They give water a higher boiling point than methane
They make water less dense than methane
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of bond holds the atoms within a water molecule together?
Metallic bond
Ionic bond
Hydrogen bond
Covalent bond
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why are covalent bonds stronger than hydrogen bonds in water?
Because they are formed between different molecules
Because they are temporary
Because they are weaker than ionic bonds
Because they involve sharing of electrons
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
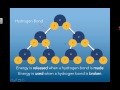
What happens when a hydrogen bond is formed?
Energy is absorbed
Energy is released
The bond becomes ionic
No energy change occurs
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Ionic vs Covalent Bonds Explained Through Real-Life Examples
Interactive video
•
9th - 10th Grade

11 questions
Properties and Behavior of Water
Interactive video
•
9th - 10th Grade

10 questions
The Unique Properties of Water and Their Biological Significance
Interactive video
•
9th - 10th Grade

11 questions
Intermolecular Forces Explained: Types and Examples
Interactive video
•
9th - 10th Grade

11 questions
Carbon Compounds and Bonding Concepts
Interactive video
•
9th - 10th Grade

11 questions
Bonding and Valence Electrons in Methane
Interactive video
•
9th - 10th Grade

11 questions
Understanding Methane Bonding and Structure
Interactive video
•
9th - 10th Grade

11 questions
Classifying Physical and Chemical Changes in Substances
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade