Fractional Distillation and Hydrocarbon Uses
Interactive Video
•
Science, Chemistry, Physics
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
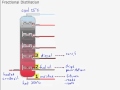
What is the temperature at the bottom of the fractional distillation tower?
500 degrees centigrade
350 degrees centigrade
25 degrees centigrade
100 degrees centigrade
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which fraction is used as bitumen for roads and roofs?
Kerosene
Diesel
Residue
Fuel oil
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary use of diesel collected during fractional distillation?
Bottle gases
Fuel for cars, buses, and trucks
Chemical production
Aircraft fuel
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which fraction is used as aircraft fuel?
Naphtha
Kerosene
Petrol
Residue
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is naphtha primarily used for?
Bottle gases
Road construction
Fuel for ships
Making chemicals
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What characteristic do small molecules at the top of the tower have?
Do not ignite easily
High boiling point
Low volatility
Flow easily
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do large molecules at the bottom of the tower not ignite easily?
They have a high boiling point
They are very volatile
They have a low boiling point
They flow easily
Similar Resources on Wayground

2 questions
GCSE Chemistry - Addition Reactions of Alkenes #55
Interactive video
•
9th - 10th Grade

6 questions
Fractional Distillation
Interactive video
•
10th Grade

8 questions
GCSE Chemistry - Crude Oil and Fractional Distillation #53
Interactive video
•
9th - 10th Grade

6 questions
Dinitrogen
Interactive video
•
9th - 10th Grade

6 questions
Dinitrogen
Interactive video
•
9th - 10th Grade

11 questions
Hydrocarbons and Fractional Distillation
Interactive video
•
9th - 10th Grade

11 questions
Understanding Fractional Distillation and Hydrocarbon Fractions
Interactive video
•
7th - 10th Grade

11 questions
Hydrocarbon Distillation Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

10 questions
Chaffey
Quiz
•
9th - 12th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

22 questions
6-8 Digital Citizenship Review
Quiz
•
6th - 8th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade
Discover more resources for Science

15 questions
Ecological Levels of Organization Quiz
Quiz
•
9th - 12th Grade

17 questions
Lab Safety
Interactive video
•
10th Grade

20 questions
Biology Lab Safety Quiz
Quiz
•
9th - 12th Grade

34 questions
H Science Basics Quiz Review
Quiz
•
9th Grade

25 questions
Lab Safety Rules and Guidelines
Quiz
•
9th Grade

15 questions
Earth Vocab Quiz 1A
Quiz
•
10th Grade

40 questions
Environmental Science Pretest
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz
Quiz
•
10th - 12th Grade