What are the two main topics covered in this lecture on air pollution?
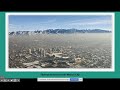
Acid Deposition and Air Pollution
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Noise pollution and light pollution
Water pollution and soil erosion
Global warming and deforestation
Photochemical smog and acid deposition
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which compound is primarily responsible for the formation of photochemical smog?
Carbon dioxide
Nitrogen oxides
Methane
Sulfur dioxide
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What role do VOCs play in the formation of photochemical smog?
They strengthen the bond with NO, reducing ozone formation
They have no effect on smog formation
They decrease the formation of smog
They increase the amount of sunlight
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In which type of areas is photochemical smog most prevalent?
Desert regions
Mountainous regions
Urban areas with high sunlight
Rural areas
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a thermal inversion and how does it affect air pollution?
A layer of warm air trapping pollutants near the ground
A decrease in temperature that clears the air
A rise in temperature that disperses pollutants
A natural phenomenon that reduces pollution
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the difference between primary and secondary pollutants in the context of acid deposition?
Primary pollutants are gases, secondary are solids
Primary pollutants are harmless, secondary are harmful
Primary pollutants are emitted directly, secondary form in the atmosphere
Primary pollutants are natural, secondary are man-made
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How has acid deposition improved over the years?
Due to global warming
By natural reduction of pollutants
Through stricter pollution control measures
Due to increased industrial activity
Create a free account and access millions of resources
Similar Resources on Quizizz

11 questions
Air Pollution and Its Effects
Interactive video
•
9th - 10th Grade

11 questions
Air Pollution and Health Effects
Interactive video
•
9th - 10th Grade

11 questions
Acid-Base Chemistry and Air Pollution
Interactive video
•
9th - 10th Grade

11 questions
Air Pollution and Health Effects
Interactive video
•
9th - 10th Grade

11 questions
Air Pollution Hazards and Solutions in Environmental Science
Interactive video
•
9th - 12th Grade

11 questions
Air Pollution and Its Effects
Interactive video
•
9th - 10th Grade

11 questions
Air Pollution: Causes, Effects, and Solutions
Interactive video
•
9th - 10th Grade

11 questions
Air Pollution and Its Effects
Interactive video
•
9th - 10th Grade
Popular Resources on Quizizz

15 questions
Multiplication Facts
Quiz
•
4th Grade

20 questions
Math Review - Grade 6
Quiz
•
6th Grade

20 questions
math review
Quiz
•
4th Grade

5 questions
capitalization in sentences
Quiz
•
5th - 8th Grade

10 questions
Juneteenth History and Significance
Interactive video
•
5th - 8th Grade

15 questions
Adding and Subtracting Fractions
Quiz
•
5th Grade

10 questions
R2H Day One Internship Expectation Review Guidelines
Quiz
•
Professional Development

12 questions
Dividing Fractions
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Spanish preterite verbs (irregular/changed)
Quiz
•
9th - 10th Grade

10 questions
Identify Slope and y-intercept (from equation)
Quiz
•
8th - 9th Grade

10 questions
Juneteenth: History and Significance
Interactive video
•
7th - 12th Grade

8 questions
"Keeping the City of Venice Afloat" - STAAR Bootcamp, Day 1
Quiz
•
9th - 12th Grade

26 questions
June 19th
Quiz
•
4th - 9th Grade

20 questions
Distance, Midpoint, and Slope
Quiz
•
10th Grade

20 questions
Figurative Language Review
Quiz
•
10th Grade

27 questions
STAAR English 1 Review
Quiz
•
9th Grade