Lone Pairs and Molecular Geometry
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Emma Peterson
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
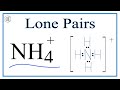
What is the charge of the ammonium ion (NH4+)?
Negative
Neutral
Variable
Positive
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of brackets in the Lewis structure of NH4+?
They indicate the presence of lone pairs.
They show the ion's charge.
They are used for aesthetic purposes.
They highlight the central atom.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many lone pairs are present in the ammonium ion (NH4+)?
Three
None
Two
One
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which molecule contains a lone pair, NH4+ or NH3?
NH4+
NH3
Both
Neither
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What effect does the lone pair in NH3 have on its molecular geometry?
It creates a trigonal planar shape.
It has no effect on the shape.
It results in a trigonal pyramidal shape.
It makes the molecule linear.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In NH3, what role does the lone pair play in relation to the hydrogen atoms?
It has no interaction with them.
It repels them upwards.
It pushes them down.
It attracts them closer.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary difference in electron arrangement between NH4+ and NH3?
NH4+ has more lone pairs.
NH3 has a lone pair while NH4+ does not.
NH3 has fewer covalent bonds.
NH4+ has a different central atom.
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

9 questions
Solubility of Magnesium Hydroxide
Interactive video
•
9th - 10th Grade

6 questions
Graphing Calculator and Linear Equations Quiz
Interactive video
•
9th - 10th Grade

6 questions
DNA Sequencing in Space Quiz
Interactive video
•
9th - 10th Grade

6 questions
A breakthrough in Eye Surgery through New Implant
Interactive video
•
9th - 10th Grade

6 questions
VOICED : Romanias oldest mine closes doors
Interactive video
•
9th - 10th Grade

7 questions
Chemical Reactions and Ionic Compounds
Interactive video
•
9th - 10th Grade

8 questions
Formal Charges and Lewis Structures
Interactive video
•
9th - 10th Grade

4 questions
Chemistry Course Engagement Quiz
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

20 questions
Periodic Table & Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

24 questions
Identifying Types of Chemical Reactions
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

10 questions
Identifying Types of Chemical Reactions
Interactive video
•
6th - 10th Grade