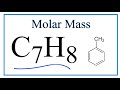
Chemical Composition and Molar Mass
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Sophia Harris
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for toluene?
C8H10
C7H8
C6H6
C5H5
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many carbon atoms are present in C7H8?
5
8
7
6
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic mass of carbon used in the calculation?
16.00 g/mol
14.01 g/mol
1.01 g/mol
12.01 g/mol
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many hydrogen atoms are present in C7H8?
8
9
7
6
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic mass of hydrogen used in the calculation?
1.01 g/mol
12.01 g/mol
14.01 g/mol
16.00 g/mol
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the calculated molar mass of C7H8?
94.12 g/mol
92.15 g/mol
96.18 g/mol
90.08 g/mol
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the molar mass of a compound represent?
The weight of one mole of the compound
The number of atoms in a molecule
The volume of the compound
The density of the compound
Similar Resources on Wayground

11 questions
Balancing Redox Reactions Concepts
Interactive video
•
9th - 12th Grade

8 questions
Autoimmune Disease & Allergies
Interactive video
•
11th Grade

11 questions
Differentiating Vectors and Scalars
Interactive video
•
9th - 12th Grade

11 questions
Understanding Limits in Calculus
Interactive video
•
9th - 12th Grade

7 questions
Understanding Urea: Molar Mass and Composition
Interactive video
•
9th - 10th Grade

8 questions
Converting Moles to Grams
Interactive video
•
9th - 10th Grade

9 questions
Nilai-nilai pancasila dalam kehidupan sehari-hari
Interactive video
•
10th Grade

6 questions
Selling the Iraq War
Interactive video
•
11th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade