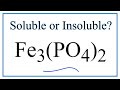
Solubility of Iron II Phosphate
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Ethan Morris
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the solubility status of Iron II Phosphate in water?
Soluble
Partially soluble
Highly soluble
Insoluble
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to solubility rules, which of the following is true about phosphates?
Phosphates dissolve in all conditions
All phosphates are soluble
Most phosphates are insoluble
Phosphates are always exceptions
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is Iron II Phosphate considered insoluble according to solubility rules?
It does not fall under any exceptions
It dissolves completely
It reacts with water
It is an exception to the rules
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'I' in the solubility chart indicate for Iron II Phosphate?
Iron II Phosphate is highly reactive
Iron II Phosphate is partially soluble
Iron II Phosphate is insoluble
Iron II Phosphate is soluble
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does Iron II Phosphate behave when placed in water according to the solubility chart?
It floats on the surface
It reacts violently
It sinks to the bottom
It dissolves completely
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of a small amount of Iron II Phosphate dissolving in water?
It indicates a chemical reaction
It is still considered insoluble
It makes it soluble
It changes the water color
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What practical method can be used to test the solubility of Iron II Phosphate?
Heating it
Mixing it with acid
Placing it in water
Exposing it to sunlight
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What would you expect to observe if you placed Iron II Phosphate in water?
It sinks to the bottom
It dissolves completely
It floats on the surface
It remains unchanged
Similar Resources on Wayground

6 questions
Solubility of Phosphates in Water
Interactive video
•
9th - 10th Grade

6 questions
Solubility of Phosphates and Compounds
Interactive video
•
9th - 10th Grade

8 questions
Solubility of Copper II Phosphate
Interactive video
•
9th - 10th Grade

8 questions
Solubility of Magnesium Phosphate
Interactive video
•
9th - 10th Grade

6 questions
Solubility of Zinc Chloride
Interactive video
•
9th - 10th Grade

9 questions
Solubility of Potassium Phosphate
Interactive video
•
9th - 10th Grade

10 questions
Chemical Compounds and Solubility Concepts
Interactive video
•
9th - 10th Grade

7 questions
Chemical Properties of Iron Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade