What is the product formed when magnesium reacts with oxygen?
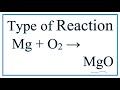
Chemical Reactions and Ionic Bonds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Magnesium nitrate
Magnesium sulfate
Magnesium oxide
Magnesium chloride
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to balance a chemical equation?
To conserve mass and atoms
To ensure the reaction is fast
To make the reaction colorful
To increase the temperature
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of reaction is Mg + O2 -> MgO?
Single displacement
Double displacement
Decomposition
Combination
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a combination reaction, how do the reactants interact?
They dissolve in water
They exchange partners
They combine to form a single product
They break apart
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of magnesium in an ionic bond?
1+
2+
1-
2-
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of oxygen in an ionic bond?
1-
2+
1+
2-
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do the charges of magnesium and oxygen cancel out in MgO?
Because they are neutral
Because they are both negative
Because they are equal and opposite
Because they are both positive
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the net charge of the product MgO?
1+
1-
2+
0
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final type of reaction for Mg + O2 -> MgO?
Double displacement
Single displacement
Combination
Decomposition
Similar Resources on Quizizz

8 questions
Ionic Compounds and Their Properties
Interactive video
•
9th - 10th Grade

9 questions
Ionic Compounds and Electronegativity
Interactive video
•
9th - 10th Grade

7 questions
Understanding Magnesium Sulfate Compounds
Interactive video
•
9th - 10th Grade

11 questions
Balancing Chemical Equations and Reactions
Interactive video
•
9th - 10th Grade

7 questions
Ionic Compounds and Nomenclature
Interactive video
•
9th - 10th Grade

8 questions
Naming and Formulas of Ionic Compounds
Interactive video
•
9th - 10th Grade

8 questions
Properties and Composition of MgCO3
Interactive video
•
9th - 10th Grade

7 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Quizizz

15 questions
Multiplication Facts
Quiz
•
4th Grade

25 questions
SS Combined Advisory Quiz
Quiz
•
6th - 8th Grade

40 questions
Week 4 Student In Class Practice Set
Quiz
•
9th - 12th Grade

40 questions
SOL: ILE DNA Tech, Gen, Evol 2025
Quiz
•
9th - 12th Grade

20 questions
NC Universities (R2H)
Quiz
•
9th - 12th Grade

15 questions
June Review Quiz
Quiz
•
Professional Development

20 questions
Congruent and Similar Triangles
Quiz
•
8th Grade

25 questions
Triangle Inequalities
Quiz
•
10th - 12th Grade
Discover more resources for Chemistry

40 questions
Week 4 Student In Class Practice Set
Quiz
•
9th - 12th Grade

40 questions
SOL: ILE DNA Tech, Gen, Evol 2025
Quiz
•
9th - 12th Grade

20 questions
NC Universities (R2H)
Quiz
•
9th - 12th Grade

25 questions
Triangle Inequalities
Quiz
•
10th - 12th Grade

46 questions
Biology Semester 1 Review
Quiz
•
10th Grade

24 questions
LSO - Virus, Bacteria, Classification - sol review 2025
Quiz
•
9th Grade

65 questions
MegaQuiz v2 2025
Quiz
•
9th - 12th Grade

10 questions
GPA Lesson
Lesson
•
9th - 12th Grade