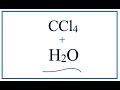
Properties and Behavior of Carbon Tetrachloride and Water
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the state of both carbon tetrachloride and water when mixed?
Both are gases
One is a liquid and the other is a gas
Both are liquids
Both are solids
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of compounds are carbon tetrachloride and water?
Ionic compounds
Molecular compounds
Metallic compounds
Covalent network solids
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is carbon tetrachloride considered nonpolar?
It has a high electronegativity difference
It has a symmetrical structure
It has a distinct positive side
It has a distinct negative side
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the shape of the carbon tetrachloride molecule?
Asymmetrical
Bent
Linear
Symmetrical
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What makes water a polar molecule?
Its symmetrical shape
Its nonpolar bonds
Its distinct positive and negative sides
Its metallic bonds
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the arrangement of hydrogen atoms in a water molecule?
They are evenly distributed
They are at the top
They are at the center
They are at the bottom
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do carbon tetrachloride and water not mix?
They have different polarities
They are both nonpolar
They have similar polarities
They are both polar
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Benzoic Acid Solubility and Polarity
Interactive video
•
9th - 10th Grade

8 questions
Naming and Properties of SnCl4
Interactive video
•
9th - 10th Grade

7 questions
Chloroform Chemistry Concepts
Interactive video
•
9th - 10th Grade

6 questions
Acids and Bases Quiz
Interactive video
•
9th - 10th Grade

7 questions
Molar Mass and Chemical Composition
Interactive video
•
9th - 10th Grade

8 questions
Balancing Chemical Reactions
Interactive video
•
9th - 10th Grade

7 questions
Lewis Structures and Valence Electrons
Interactive video
•
9th - 10th Grade

11 questions
Binary Covalent Compounds Quiz
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade