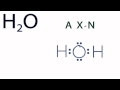
Molecular Geometry of Water
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in determining the molecular geometry of H2O?
Using a 3D model
Consulting a table
Examining the Lewis structure
Applying the AXN notation
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to the valence shell electron pair repulsion theory, how do electron pairs arrange themselves?
As far apart as possible
In a circular pattern
In a linear fashion
As close as possible
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What shape does the water molecule have when visualized in three dimensions?
Linear
Bent
Trigonal planar
Tetrahedral
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the AXN notation, what does 'A' represent?
The central atom
The bond angle
The number of attached atoms
The number of lone pairs
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many non-bonding electron pairs are present in the water molecule according to the AXN notation?
Four
Three
Two
One
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of H2O as determined by the AXN notation?
Trigonal planar
Tetrahedral
Linear
Bent
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What bond angle is associated with the bent molecular geometry of water?
90°
120°
109.5°
180°
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Molecular Geometry of H2O2
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry and Bond Angles
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry and AXN Notation
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry and AXN Notation
Interactive video
•
9th - 10th Grade

8 questions
Molecular Geometry of CO2
Interactive video
•
9th - 10th Grade

9 questions
Molecular Geometry of N2O
Interactive video
•
9th - 10th Grade

7 questions
Geometry and Bonding in SO2
Interactive video
•
9th - 10th Grade

10 questions
Acetylene Molecular Geometry and Bonding
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

11 questions
Hallway & Bathroom Expectations
Quiz
•
6th - 8th Grade

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade
Discover more resources for Chemistry

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

24 questions
Scientific method and variables review
Quiz
•
9th Grade

10 questions
Characteristics of Life
Quiz
•
9th - 10th Grade

19 questions
Mental Health Vocabulary Pre-test
Quiz
•
9th Grade

14 questions
Points, Lines, Planes
Quiz
•
9th Grade