Comparing Chemical Properties and Structures
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Emma Peterson
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
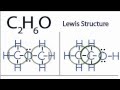
What is the name of the compound when the oxygen atom is placed between the two carbon atoms in the C2H6O structure?
Ethanol
Propane
Dimethyl ether
Methanol
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which compound is formed when the oxygen atom is on the outside with hydrogen attached in the C2H6O structure?
Dimethyl ether
Ethanol
Acetone
Butanol
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What term is used to describe compounds with the same chemical formula but different structures?
Isomers
Allotropes
Isotopes
Polymers
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do the chemical and physical properties of dimethyl ether and ethanol compare?
They are identical
They are similar
They are different
They are unrelated
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons do carbon atoms have in the C2H6O Lewis structures?
Eight
Four
Two
Six
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the required number of valence electrons for hydrogen to have a full outer shell?
Eight
Two
Four
Six
Similar Resources on Wayground

7 questions
Valence Electrons in CH3SH
Interactive video
•
9th - 10th Grade

6 questions
Beryllium and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in S2Cl2 Structure
Interactive video
•
9th - 10th Grade

6 questions
BrO- Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in H3O+
Interactive video
•
9th - 10th Grade

6 questions
B(OH)3 Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in PI3 Molecule
Interactive video
•
9th - 10th Grade

7 questions
Phosphorus and PBr5 Valence Electrons
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade