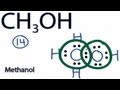
Lewis Structure of Methanol
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the position of the OH group in the Lewis structure of Methanol?
In the center of the molecule
Attached directly to the Carbon
Attached to the Hydrogen
Floating freely
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are present in the Lewis structure of CH3OH?
14
12
16
10
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in forming chemical bonds in the Lewis structure of Methanol?
Placing all electrons on Oxygen
Adding electrons to Hydrogen first
Distributing electrons equally among all atoms
Putting 2 electrons between atoms to form bonds
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does Oxygen have in the completed Lewis structure of Methanol?
8
6
12
10
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons used in the Lewis structure of Methanol?
10
12
16
14
Similar Resources on Wayground

6 questions
Lewis Structure of Methylamine
Interactive video
•
9th - 10th Grade

6 questions
Boron and Fluorine Chemistry Concepts
Interactive video
•
9th - 10th Grade

6 questions
Boron and BH4- Lewis Structure
Interactive video
•
9th - 10th Grade

6 questions
Silicon Valence Electrons and Structures
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in Methanol
Interactive video
•
9th - 10th Grade

6 questions
Balancing Chemical Equations and Products
Interactive video
•
9th - 10th Grade

10 questions
Covalent Compounds and Molecular Models
Interactive video
•
9th - 10th Grade

6 questions
Understanding OH- Lewis Structure
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade