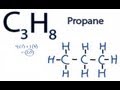
Lewis Structure of Propane
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Jackson Turner
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many total valence electrons are present in the Lewis structure of Propane (C3H8)?
22
20
24
18
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the Lewis structure of Propane, how are the Carbon atoms arranged?
In a zigzag pattern
In a triangular shape
In a circular formation
In a straight line
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many Hydrogen atoms are bonded to each end Carbon atom in Propane?
1
2
4
3
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of a single bond in the Lewis structure of Propane?
It represents 1 valence electron
It represents 4 valence electrons
It represents 2 valence electrons
It represents 3 valence electrons
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many single bonds does each Carbon atom form in the Propane molecule?
2
3
4
5
Similar Resources on Wayground

6 questions
Die Geschichte vom Suppen-Kaspar - Märchen - Deutsch lernen
Interactive video
•
10th - 12th Grade

6 questions
Follies in Sammy's
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : New Mali junta opens talks on transition to civilian rule
Interactive video
•
9th - 10th Grade

2 questions
The Heart Anatomy Song
Interactive video
•
KG - 9th Grade

8 questions
Dianne Bilyak "A Bell of Water, Ringing"
Interactive video
•
9th - 10th Grade

6 questions
I WONDER - Are There Different Parts To The Intestine? Me Pregunto - Existen Diferentes Partes En Los Intestinos?
Interactive video
•
KG - 12th Grade

2 questions
How to get funding for your public health project.
Interactive video
•
10th - 12th Grade

2 questions
Ancient Rome - The Dynasties Of Rome
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Forest Self-Management
Lesson
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

30 questions
Thanksgiving Trivia
Quiz
•
9th - 12th Grade

30 questions
Thanksgiving Trivia
Quiz
•
6th Grade

11 questions
Would You Rather - Thanksgiving
Lesson
•
KG - 12th Grade

48 questions
The Eagle Way
Quiz
•
6th Grade

10 questions
Identifying equations
Quiz
•
KG - University

10 questions
Thanksgiving
Lesson
•
5th - 7th Grade
Discover more resources for Chemistry

88 questions
Test Review
Quiz
•
9th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

22 questions
Unit 2 Part 1 Rumble
Quiz
•
10th Grade

20 questions
Molar Mass
Quiz
•
9th - 12th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade