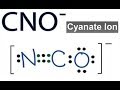
CNO- Lewis Structure and Valence Electrons
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many total valence electrons are present in the CNO- ion?
20
14
16
18
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center of the CNO- Lewis structure?
Carbon
Oxygen
Hydrogen
Nitrogen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial number of valence electrons around Carbon before sharing with Nitrogen?
8
2
4
6
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does Nitrogen have after sharing with Carbon to form a triple bond?
8
7
6
9
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is a triple bond formed between Carbon and Nitrogen in the CNO- structure?
To increase the number of valence electrons
To make the structure symmetrical
To satisfy the octet rule for Carbon
To decrease the formal charge on Oxygen
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formal charge on Oxygen in the CNO- Lewis structure?
0
-1
+1
-2
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What would be the result of forming a double bond between Carbon and Oxygen?
The octet rule would not be satisfied for Carbon
The structure would have a negative charge
All atoms would have a formal charge of 0
Oxygen would have a positive formal charge
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the arrangement with a triple bond between Carbon and Nitrogen preferred?
It gives each atom a formal charge of 0
It aligns with the electronegativity of the atoms
It results in the lowest energy state
It maximizes the number of bonds
Similar Resources on Wayground

7 questions
Valence Electrons in N2H4
Interactive video
•
9th - 10th Grade

6 questions
C2H2Br2 Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
Lewis Structure of Methanol
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in IBr Molecule
Interactive video
•
9th - 10th Grade

6 questions
SF6 Lewis Structure and Valence Electrons
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
Lewis Structures and Molecular Geometry
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade