Hydrocarbon Structures and Valence Electrons
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
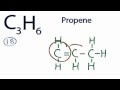
How many valence electrons are present in the C3H6 molecule?
22
16
18
20
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the shape of the cyclopropane structure?
Ring
Bent
Tetrahedral
Linear
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the cyclopropane structure, how many single bonds does each carbon atom have?
Two
Five
Three
Four
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the other possible structure for C3H6 besides cyclopropane?
Propene
Butane
Ethene
Methane
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the propene structure, how many valence electrons are used?
18
16
22
20
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is true about the hydrogen atoms in both cyclopropane and propene structures?
They form triple bonds.
They have incomplete outer shells.
They have two valence electrons.
They have double bonds.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What can be concluded about the C3H6 formula in terms of its Lewis structures?
Only cyclopropane is correct.
Only propene is correct.
Both cyclopropane and propene are correct.
Neither structure is correct.
Similar Resources on Wayground

7 questions
Valence Electrons in CH3SH
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in PI3 Molecule
Interactive video
•
9th - 10th Grade

7 questions
Phosphorus and PBr5 Valence Electrons
Interactive video
•
9th - 10th Grade

6 questions
BrO- Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in S2Cl2 Structure
Interactive video
•
9th - 10th Grade

6 questions
Beryllium and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
B(OH)3 Lewis Structure Concepts
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in H3O+
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade