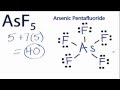
Arsenic in AsF5 Lewis Structure
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Olivia Brooks
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does Arsenic contribute in the AsF5 Lewis structure?
5
9
3
7
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in the AsF5 molecule?
40
35
45
30
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where is Arsenic placed in the AsF5 Lewis structure?
At the bottom
At the center
On the side
At the top
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many electrons are used in forming chemical bonds between Arsenic and Fluorine?
20
15
10
5
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why can Arsenic have more than 8 valence electrons in AsF5?
It is a noble gas
It is in period 3 of the periodic table
It is in period 4 of the periodic table
It is a metal
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formal charge on the atoms in the AsF5 Lewis structure?
0
+2
-1
+1
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade