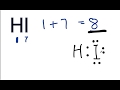
Valence Electrons and Bonding Concepts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in hydroiodic acid?
6
7
8
9
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which group does iodine belong to on the periodic table?
Group 2
Group 7 or 17
Group 6
Group 1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to bond hydrogen and iodine in hydroiodic acid?
6
8
4
2
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the full outer shell requirement for hydrogen?
8 electrons
6 electrons
2 electrons
4 electrons
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does iodine have in its outer shell after bonding?
8
6
4
2
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the structural formula of hydroiodic acid represent?
Triple bond
A single bond
A double bond
No bond
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of the Lewis structure in chemistry?
To represent the arrangement of electrons
To show the atomic number
To indicate the melting point
To display the atomic mass
Similar Resources on Wayground

6 questions
Valence Electrons in CBr4
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in PCl3
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in CH2F2
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
SF2 Lewis Structure and Valence Electrons
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in SiCl4
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in IBr Molecule
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Molecular Structure
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

11 questions
Hallway & Bathroom Expectations
Quiz
•
6th - 8th Grade

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade
Discover more resources for Chemistry

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

24 questions
Scientific method and variables review
Quiz
•
9th Grade

10 questions
Characteristics of Life
Quiz
•
9th - 10th Grade

19 questions
Mental Health Vocabulary Pre-test
Quiz
•
9th Grade

14 questions
Points, Lines, Planes
Quiz
•
9th Grade