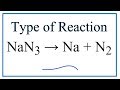
Decomposition Reactions in Chemistry
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Ethan Morris
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of reaction is demonstrated by sodium nitride breaking down into sodium and nitrogen gas?
Decomposition Reaction
Synthesis Reaction
Single Replacement Reaction
Double Replacement Reaction
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a decomposition reaction, a compound breaks down into:
Two or more simpler substances
A single more complex substance
Two identical substances
A mixture of elements and compounds
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following represents the general form of a decomposition reaction?
AB + C → AC + B
A + B → C
AB → A + B
A + B → AB
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for sodium nitride?
NaN3
Na2N
Na3N
NaN
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where can you find additional help for balancing the decomposition reaction?
In the video description
In the video title
In the video comments
In the video thumbnail
Similar Resources on Wayground

7 questions
Balancing Chemical Reactions with Sodium
Interactive video
•
9th - 10th Grade

6 questions
Nitride Ion and Nickel Compounds
Interactive video
•
9th - 10th Grade

7 questions
Chemical Reactions and Balancing Equations
Interactive video
•
9th - 10th Grade

6 questions
Barium Chloride and Sodium Sulfate Reaction
Interactive video
•
8th - 10th Grade

9 questions
Balancing Chemical Reactions
Interactive video
•
9th - 10th Grade

6 questions
Decomposition Reactions in Chemistry
Interactive video
•
9th - 10th Grade

6 questions
Dinitrogen
Interactive video
•
9th - 10th Grade

8 questions
Solubility and Ionic Reactions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade