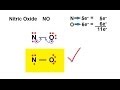
Nitrous Oxide Lewis Structure Concepts
Interactive Video
•
Chemistry, Science, Biology
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a key reason for exceptions to the octet rule in elements from the third or fourth period?
They are less reactive.
They are more electronegative.
They have fewer than eight valence electrons.
They have more than eight valence electrons.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is nitrous oxide unable to satisfy the octet rule?
It has an odd number of valence electrons.
It has an even number of valence electrons.
It is too stable.
It is too reactive.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in nitrous oxide?
Ten
Thirteen
Twelve
Eleven
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the initial Lewis structure attempt for nitrous oxide, how many valence electrons are around the nitrogen atom?
Four
Six
Five
Seven
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of electronegativity in determining the central atom in a Lewis structure?
The most electronegative atom is central.
The least electronegative atom is central.
The central atom is always oxygen.
Electronegativity does not affect the central atom.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens when a double bond is introduced in the Lewis structure of nitrous oxide?
Both atoms follow the octet rule.
Oxygen follows the octet rule.
Neither atom follows the octet rule.
Nitrogen follows the octet rule.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom in nitrous oxide is more electronegative?
Nitrogen
Oxygen
Both are equally electronegative
Neither is electronegative
Create a free account and access millions of resources
Similar Resources on Wayground

8 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

7 questions
NO2 Structure and Valence Electrons
Interactive video
•
9th - 10th Grade

8 questions
Valence Electrons in BH2- Structure
Interactive video
•
9th - 10th Grade

8 questions
Aluminum and Valence Electrons Concepts
Interactive video
•
9th - 10th Grade

8 questions
Valence Electrons in CHBr3
Interactive video
•
9th - 10th Grade

7 questions
Valence Electrons in CH3SH
Interactive video
•
9th - 10th Grade

11 questions
Resonance Structures and Formal Charges
Interactive video
•
9th - 10th Grade

8 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade