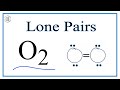
Lone Pairs and Diatomic Oxygen
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are lone pairs in the context of diatomic oxygen (O2)?
Neutrons in the nucleus
Electrons involved in chemical bonds
Electrons not involved in chemical bonds
Protons in the nucleus
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where is diatomic oxygen (O2) commonly found?
In the atmosphere
In the Earth's core
In the ocean
In the Earth's crust
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the difference between lone pairs and bonding pairs of electrons?
Both are shared between atoms
Bonding pairs are shared between atoms, lone pairs are not
Neither are shared between atoms
Lone pairs are shared between atoms, bonding pairs are not
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many lone pairs are present in O2?
Five
Four
Three
Two
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a Lewis structure, how are bonding pairs of electrons typically represented?
With circles
With dots
With lines
With arrows
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many pairs of electrons are involved in the double bond of O2?
Three pairs
Four pairs
One pair
Two pairs
Similar Resources on Wayground

8 questions
Lone Pairs and Molecular Geometry
Interactive video
•
9th - 10th Grade

8 questions
Sulfate Ion Properties and Characteristics
Interactive video
•
9th - 10th Grade

8 questions
BF3 Molecular Geometry and Electron Pairs
Interactive video
•
9th - 10th Grade

9 questions
Molecular Geometry of Methane
Interactive video
•
9th - 10th Grade

8 questions
Covalent Compounds and PCl3 Structure
Interactive video
•
9th - 10th Grade

7 questions
Bromine and Fluorine Bonding Concepts
Interactive video
•
9th - 10th Grade

7 questions
NO2 Structure and Valence Electrons
Interactive video
•
9th - 10th Grade

8 questions
Lone Pairs and Molecular Geometry
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

11 questions
Hallway & Bathroom Expectations
Quiz
•
6th - 8th Grade

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade
Discover more resources for Chemistry

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

24 questions
Scientific method and variables review
Quiz
•
9th Grade

10 questions
Characteristics of Life
Quiz
•
9th - 10th Grade

19 questions
Mental Health Vocabulary Pre-test
Quiz
•
9th Grade

14 questions
Points, Lines, Planes
Quiz
•
9th Grade