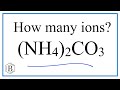
Ammonium Carbonate and Ion Chemistry
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Liam Anderson
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of the carbonate ion?
One minus
One plus
Two minus
Two plus
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ion is represented by NH4+?
Carbonate ion
Ammonium ion
Sulfate ion
Nitrate ion
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to ammonium carbonate when it is dissolved in water?
It forms a solid precipitate
It dissolves and forms aqueous ions
It evaporates
It remains unchanged
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many moles of ammonium ions are present in one mole of ammonium carbonate?
Four moles
Three moles
Two moles
One mole
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If you have one mole of ammonium carbonate, how many moles of carbonate ions do you have?
One mole
Four moles
Two moles
Three moles
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Given 0.43 moles of ammonium carbonate, how many moles of ammonium ions are present?
1.29 moles
0.43 moles
0.86 moles
0.21 moles
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many moles of carbonate ions are present in 0.43 moles of ammonium carbonate?
0.86 moles
0.43 moles
0.21 moles
1.29 moles
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of ions produced when ammonium carbonate dissolves in water?
One ion
Two ions
Four ions
Three ions
Similar Resources on Wayground

11 questions
Ions and Their Charges
Interactive video
•
9th - 10th Grade

6 questions
Ions and Atoms in NH4(3PO3)
Interactive video
•
9th - 10th Grade

8 questions
Ammonium Bicarbonate and Ionic Compounds
Interactive video
•
9th - 10th Grade

6 questions
Understanding Ammonium Chromate Composition
Interactive video
•
9th - 10th Grade

9 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

8 questions
Ammonium Sulfate and Its Properties
Interactive video
•
9th - 10th Grade

10 questions
Solubility of Ammonium Carbonate
Interactive video
•
9th - 10th Grade

8 questions
Chemical Composition and Properties of K2SO4
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade